
As filed with the Securities and Exchange Commission on July 21, 2010
Registration No. 333–163957
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
AMENDMENT NO. 11
TO
FORM S–1
REGISTRATION STATEMENT
UNDER
THE SECURITIES ACT OF 1933
SurgiVision, Inc.
(Exact name of registrant as specified in its charter)
| Delaware | 3841 | 58-2394628 | ||
| (State or other jurisdiction of incorporation or organization) |
(Primary Standard Industrial Classification Code Number) |
(I.R.S. Employer Identification No.) |
SurgiVision, Inc.
One Commerce Square, Suite 2550
Memphis, TN 38103
(901) 522-9300
(Address, including zip code, and telephone number, including area code, of registrant’s principal executive office)
Kimble L. Jenkins
Chief Executive Officer
SurgiVision, Inc.
One Commerce Square, Suite 2550
Memphis, TN 38103
(901) 522-9300
(Name, address, including zip code, and telephone number, including area code, of agent for service)
Copies to:
| Robert J. DelPriore, Esq. Matthew S. Heiter, Esq. Richard F. Mattern, Esq. Baker, Donelson, Bearman, Caldwell & Berkowitz, PC 165 Madison Avenue, Suite 2000 Memphis, TN 38103 (901) 577-8228 |
Carmelo M. Gordian, Esq. Edward A. Gilman, Esq. Nicholas F. Ducoff, Esq. Andrews Kurth LLP 111 Congress Avenue, Suite 1700 Austin, TX 78701 (512) 320-9290 |
Approximate date of commencement of proposed sale to the public: As soon as practicable after the effective date of this registration statement.
If any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933, check the following box. ¨
If this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, please check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ¨
If this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ¨
If this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ¨
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act. (check one)
| Large Accelerated filer |
¨ |
Accelerated filer | ¨ | |||
| Non-accelerated filer |
x (Do not check if a smaller reporting company) |
Smaller reporting company | ¨ |
| Title of Each Class of Securities to be Registered |
Amount to be Registered (1) |
Proposed Maximum per Share (2) |
Proposed Maximum Aggregate Offering Price (2) |
Amount
of Registration Fee (3) | |||||||
| Common Stock, $0.01 par value per share |
2,875,000 shares | $ | 11.00 | $ | 31,625,000 | $ | 2,255.00 | ||||
| (1) | Includes shares of common stock that the underwriters have an option to purchase. |
| (2) | This amount represents the proposed maximum aggregate offering price of the securities registered hereunder to be sold by the Registrant. These figures are estimated solely for the purpose of computing the registration fee in accordance with Rule 457(a) under the Securities Act of 1933, as amended. |
| (3) | Previously paid a registration fee of $3,075.00 on June 25, 2010. |
The Registrant hereby amends this registration statement on such date or dates as may be necessary to delay its effective date until the Registrant shall file a further amendment which specifically states that this registration statement shall thereafter become effective in accordance with Section 8(a) of the Securities Act of 1933, as amended, or until the registration statement shall become effective on such date as the Securities and Exchange Commission, acting pursuant to Section 8(a), may determine.
The information in this preliminary prospectus is not complete and may be changed. We may not sell these securities until the registration statement filed with the Securities and Exchange Commission is effective. This preliminary prospectus is not an offer to sell nor does it seek an offer to buy these securities in any jurisdiction where the offer or sale is not permitted.
| PRELIMINARY PROSPECTUS |
SUBJECT TO COMPLETION, DATED JULY 21, 2010 |

2,500,000 Shares
SurgiVision, Inc.
Common Stock
This is the initial public offering of shares of common stock of SurgiVision, Inc. We are offering 2,500,000 shares of our common stock.
No public market currently exists for our common stock. We estimate that the initial public offering price will be between $9.00 and $11.00 per share. We have been approved for the quotation of our common stock on the Nasdaq Capital Market under the symbol “SRGV”.
Investing in our common stock involves risk. See “Risk Factors” beginning on page 7 of this prospectus to read about factors you should consider before buying shares of our common stock.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or passed upon the adequacy or accuracy of this prospectus. Any representation to the contrary is a criminal offense.
| Per Share | Total | |||||
| Initial public offering price |
$ | $ | ||||
| Underwriting discounts and commissions(1) |
$ | $ | ||||
| Proceeds, before expenses, to us |
$ | $ | ||||
| (1) | See “Underwriting” beginning on page 127 of this prospectus to read about compensation to the underwriters. |
To the extent that the underwriters sell more than 2,500,000 shares of our common stock, the underwriters have the option to purchase up to an additional 375,000 shares from us at the initial public offering price less the underwriting discounts and commissions.
In connection with this offering, we have also agreed to issue to the underwriters warrants to purchase up to an aggregate of 125,000 shares of our common stock at an exercise price of $12.50 per share assuming an initial public offering price of $10.00 per share, which is the mid-point of the range listed above. These warrants are exercisable commencing on the first anniversary of the date of this prospectus and ending on the fifth anniversary of the date of this prospectus.
The underwriters expect to deliver the shares on or about , 2010.
| Canaccord Genuity |
Rodman & Renshaw, LLC |
Prospectus dated , 2010
| Page | ||
| 1 | ||
| 7 | ||
| 34 | ||
| 35 | ||
| 36 | ||
| 37 | ||
| 39 | ||
| 41 | ||
| MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS |
42 | |
| 57 | ||
| 91 | ||
| 105 | ||
| 114 | ||
| 116 | ||
| 118 | ||
| 122 | ||
| 124 | ||
| 127 | ||
| 136 | ||
| 136 | ||
| 137 | ||
| F-1 |
No dealer, salesperson or other person is authorized to give any information or to represent anything not contained in this prospectus. You must not rely on any unauthorized information or representations. This prospectus is an offer to sell only the shares of common stock offered hereby, but only under circumstances and in jurisdictions where it is lawful to do so. The information contained in this prospectus is current only as of its date.
For investors outside the United States: We have not and the underwriters have not done anything that would permit this offering or possession or distribution of this prospectus in any jurisdiction where action for that purpose is required, other than in the United States. Persons outside the United States who come into possession of this prospectus must inform themselves about, and observe any restrictions relating to, the offering of the shares of our common stock and the distribution of this prospectus outside the United States.
Dealer Prospectus Delivery Obligation
Through and including , 2010 (the 25th day after the date of this prospectus), all dealers effecting transactions in these securities, whether or not participating in this offering, may be required to deliver a prospectus. This is in addition to a dealer’s obligation to deliver a prospectus when acting as an underwriter and with respect to an unsold allotment or subscription.
i
Trademarks, Trade Names and Service Marks
ClearConnect™ , ClearPoint™, ClearTrace™, SmartFrame™, SmartGrid™ and SurgiVision™ are trademarks of SurgiVision, Inc. All other trademarks, trade names and service marks referred to in this prospectus are the property of their respective owners. Siemens refers to Siemens Aktiengesellschaft, Healthcare Sector. Boston Scientific refers to Boston Scientific Corporation and its affiliates.
Industry and Market Data
The market data and other statistical information used throughout this prospectus are based on independent industry publications, government publications, reports by market research firms and other published independent sources. Some data is also based on our good faith estimates, which are derived from other relevant statistical information, as well as the independent sources listed above. Although we believe these sources are reliable, we have not independently verified the information.
ii
This summary highlights the information contained elsewhere in this prospectus. Because this is only a summary, it does not contain all of the information that may be important to you. Before investing in our common stock, you should read this entire prospectus, including the information set forth under the heading “Risk Factors” and the financial statements and the notes thereto.
Unless the context otherwise requires, references in this prospectus to “SurgiVision,” “we,” “our,” “us” and the “company” refer to SurgiVision, Inc. The historical financial statements and financial data included in this prospectus are those of SurgiVision, Inc. and its consolidated subsidiary, which was merged into SurgiVision, Inc. on June 11, 2010.
Our Business
We are a medical device company focused on the development and commercialization of technology that enables physicians to see inside the brain and heart using direct, intra-procedural magnetic resonance imaging, or MRI, guidance while performing minimally invasive procedures. Utilizing hospitals’ existing MRI suites, we believe that our marketed products and our product candidates will deliver better patient outcomes in shorter procedure times, enhance revenue potential for both physicians and hospitals, and reduce costs to the healthcare system. For the year ended December 31, 2009, we recorded revenues of $2,600,000, incurred a net loss of approximately $7,159,000, and received a going concern qualification from our auditors. For the three months ended March 31, 2010, we generated revenues of $650,000 and incurred a net loss of approximately $2,516,000.
Millions of people suffer from brain and heart diseases and disorders. While some patients can be treated with medication, some will require surgery. Current surgical interventions include both open and minimally invasive procedures. Given the option, patients, physicians and hospitals prefer minimally invasive procedures over open procedures. However, because of restricted visibility of the patient’s anatomy, surgical field and instruments, minimally invasive alternatives for some procedures in the brain and heart are either unavailable or exceedingly complex.
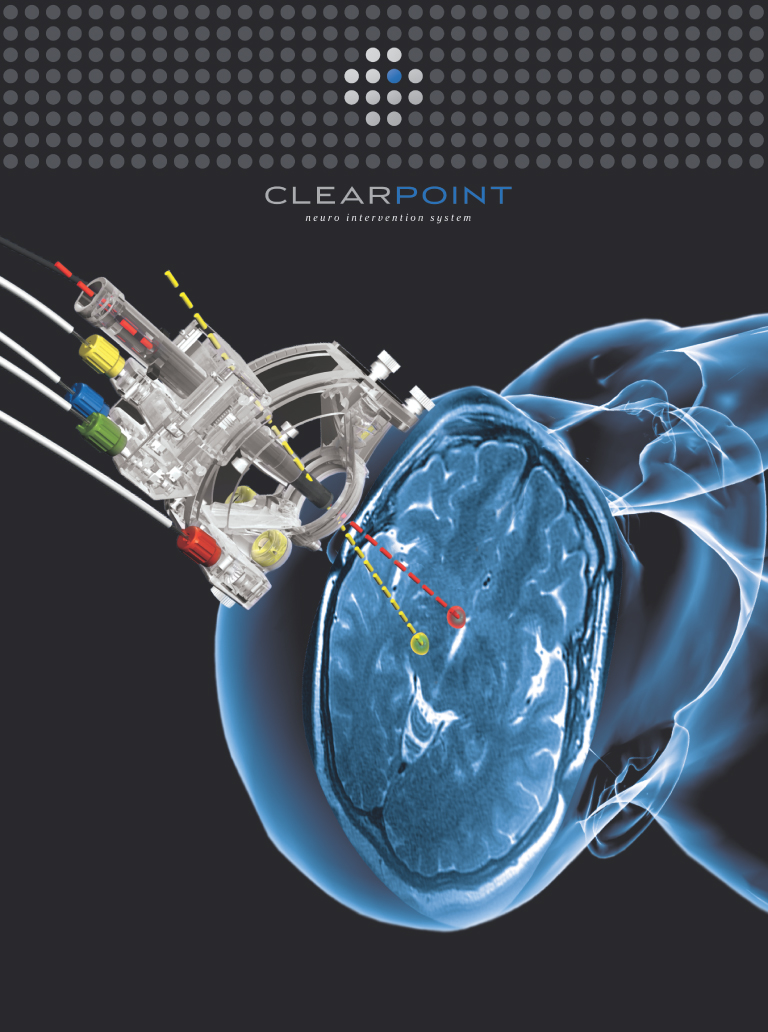
To address these issues, we have designed two innovative platforms for use in hospitals’ existing MRI suites. We call these platforms our ClearPoint system and the ClearTrace system. By combining the continuous, high resolution imaging capabilities of MRI with minimally invasive techniques, these two platforms, subject to appropriate regulatory clearance or approval, will enable physicians to:
| • | Guide a surgical instrument within the patient as it is advanced towards the therapeutic target; |
| • | Deliver a planned therapy with precise visualization of a patient’s anatomy, the surgical field and instruments; |
| • | Monitor for adverse events during and immediately after the administration of the therapy; and |
| • | Confirm the desired results of a procedure. |
Our Marketed Products
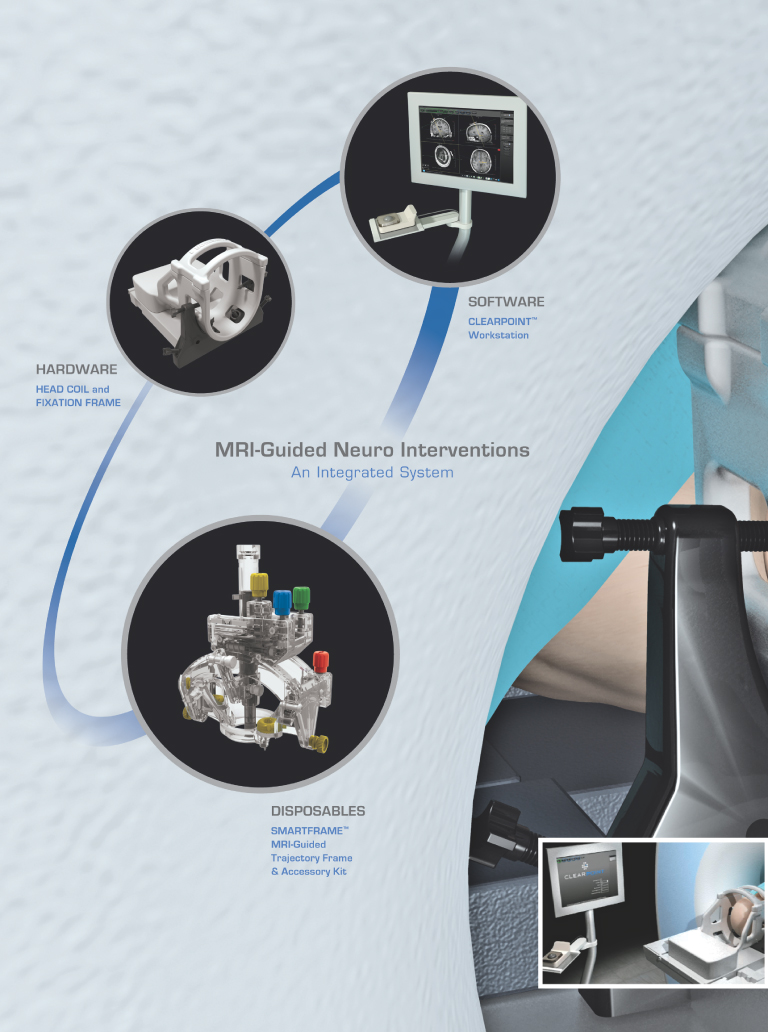
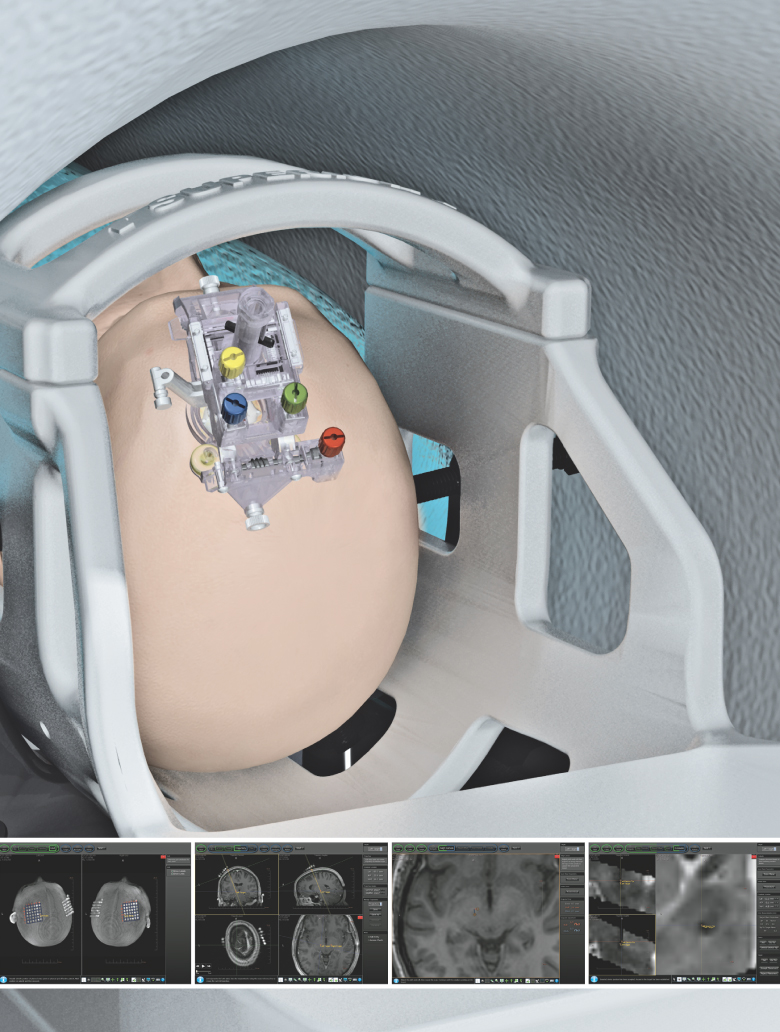
Our ClearPoint system is designed to allow minimally invasive procedures in the brain to be performed in an existing MRI suite. Our ClearPoint system is an integrated system of reusable components, disposable components and intuitive, menu-driven software, which we refer to as our marketed products. Using our ClearPoint system, a physician sees and selects a neurological target, aims our targeting device and watches as the surgical instrument is advanced to the target, significantly reducing the time and complexity of the interventional procedure. Millions of people suffer from neurological disorders or diseases. Performing minimally invasive procedures in the brain presents special challenges, including a need to reach small therapeutic targets often located deep within the brain. We believe that our ClearPoint system addresses these challenges and can become the platform-of-choice for performing the next generation of minimally invasive procedures in the brain.
1
On June 16, 2010, we received 510(k) clearance from the Food and Drug Administration, or the FDA, to market our ClearPoint system in the United States for general neurological interventional procedures. We are marketing our ClearPoint system to provide guidance for the placement and operation of instruments or devices during the planning and operation of neurological procedures within the MRI environment and in conjunction with MR imaging. Our ClearPoint system is intended to be used as an integral part of procedures such as biopsies and catheter and electrode insertion, which have traditionally been performed using other methods. We are focusing our marketing efforts on physicians and hospitals to adopt use of our ClearPoint system. Our strategy is to convince physicians that our ClearPoint system offers a better procedural solution to their patients. We will work with the physicians to encourage hospitals to install our ClearPoint system in their existing MRI suites. Once our ClearPoint system is installed in a hospital, we will focus on selling the disposable components of our ClearPoint system to generate recurring revenues. We have not yet sold a ClearPoint system and as a result, we have not generated any revenues or recurring revenues from the sale of the reusable or disposable components of the ClearPoint system.
Our Product Candidates
The following table summarizes key information about our product candidates:
| Product Candidate |
Regulatory Status |
Target Market |
Development Partner | |||
| ClearTrace Cardiac Intervention System | Development Stage | Initial target market is catheter-based cardiac ablation to treat cardiac arrhythmias, such as atrial fibrillation. Subsequent target markets may include precision delivery of drugs and biologics. | Siemens | |||
| SafeLead Development Program | Development Stage | Target market is implantable leads for cardiac and neurological applications. | Boston Scientific | |||
The ClearTrace system is designed to allow catheter-based minimally invasive procedures in the heart to be performed using continuous, intra-procedural MRI guidance. We are developing the hardware and MRI software for the ClearTrace system with Siemens, the global market leader in MRI scanners. The ClearTrace system is an integrated system of reusable components, disposable catheters and intuitive, menu-driven software. The ClearTrace system will offer a novel, comprehensive solution for the planning, delivery and intra-procedural assessment of catheter-based cardiac interventions. We expect that the ClearTrace system’s initial application will be catheter-based cardiac ablation to treat cardiac arrhythmias, such as atrial fibrillation. During cardiac ablation, a physician attempts to restore a normal heart rhythm by destroying small areas of heart tissue to block irregular electrical impulses that cause an irregular heartbeat, or arrhythmia. Atrial fibrillation is the most common cardiac arrhythmia, affecting over three million people in the United States alone.
Our other area of development activity is referred to as the SafeLead Development Program. Over the last ten years, we have pioneered several technologies that improve the MRI-safety profile of implantable medical leads. These leads are thin, insulated wires that are connected to implantable generators, such as a pacemaker or neurostimulator, and deliver electrical pulses or stimulation to a specific area of the body, such as the heart or the brain. During an MRI scan, these leads are susceptible to heating, which could burn and destroy adjacent tissue. Our technologies address this issue by maintaining lead temperatures well within safe levels during an MRI scan. We are working with Boston Scientific to incorporate our MRI-safety technologies into Boston Scientific’s implantable leads for cardiac and neurological applications. Boston Scientific paid us licensing fees of $13,000,000 in 2008 relating to implantable cardiac leads. In addition, under our agreements, Boston Scientific has agreed to pay us up to $21,600,000 in future milestone-based payments as well as royalties on net sales of products that are covered by a licensed patent. We believe that our MRI-safety technologies, when integrated into Boston Scientific’s implantable leads, could represent a meaningful market differentiator over existing implantable lead designs.
2
Licenses and Collaborative Relationships
In addition to our internally-developed technologies and devices, we have established and intend to continue to pursue licensing and collaborative relationships with medical device companies and academic institutions to further the development and commercialization of our product platforms and core technologies. Our most significant licensing and collaborative relationships are summarized below:
| • | Siemens. We have entered into an agreement with Siemens to develop the hardware and MRI software systems for MRI-guided, catheter-based cardiac ablation to treat cardiac arrhythmias, such as atrial fibrillation. Under this agreement, Siemens will develop the software, and we will develop the catheters and other hardware, other than the MRI scanner and workstation. The agreement contains exclusivity provisions in the area of MRI-guided, catheter-based cardiac ablation. These provisions prohibit Siemens from marketing or offering software intended to work with other manufacturers’ catheters. These provisions also prohibit us from selling or offering catheters intended to work with other manufacturers’ MRI scanners. |
| • | Boston Scientific. We have entered into a series of agreements with Boston Scientific with respect to our MRI-safety technologies. Under these agreements, Boston Scientific has the exclusive, worldwide right, but not the obligation, to use the licensed technologies in Boston Scientific’s implantable leads for cardiac and neurological applications. We are working jointly with Boston Scientific to assess the potential use of our MRI-safety technologies in Boston Scientific’s lead designs. |
| • | University of California, San Francisco. We have entered into a research agreement with the University of California, San Francisco in the field of interventional MRI. Under our agreement, university personnel are conducting research activities relating to interventional MRI guidance for the performance of certain minimally invasive neurological procedures, including an assessment of the safety and clinical efficacy of such procedures. |
| • | The University of Utah. We have established a collaboration with The University of Utah, under which university personnel are conducting research activities and experiments to develop knowledge, techniques, methods and technologies related to MRI-guided cardiac ablation, including a specific focus on MRI-guided cardiac ablation to treat atrial fibrillation. |
| • | The Johns Hopkins University. We have several license agreements with The Johns Hopkins University under which we have obtained exclusive licenses for various technologies relating to devices, systems and methods for performing MRI-guided interventions and MRI-safety. |
Our Business Model and Strategy
Our business model is focused on producing recurring revenue from the sale of the disposable components of both the ClearPoint and ClearTrace systems. Each system’s reusable components can be installed, at minimal cost to the hospital, without disrupting the hospital’s routine schedule for use of its MRI scanner. Our disposable and reusable components are tightly integrated, which allows us to leverage each new installation of a ClearPoint or ClearTrace system to generate recurring sales of our disposable products. We anticipate that recurring revenues will constitute an increasing percentage of our total revenues as our installed base grows.
The key elements of our business strategy are maximizing installation and adoption of our ClearPoint system, continuing development of the ClearTrace system with Siemens, pursuing the SafeLead Development Program with Boston Scientific, and building upon our core technologies to continue to develop additional MRI-based products.
We have a significant intellectual property portfolio in the field of MRI-guided interventions. As of April 30, 2010, our portfolio included 40 patents and 115 patent applications, both United States and foreign, which we wholly-own, co-own or have licensed. In addition, we have meaningful collaborations with major industry participants and renowned academic institutions. Our technologies have been the subject of numerous peer-
3
reviewed articles in medical and scientific journals. As a result of our intellectual property and collaborative relationships, we believe that we are well positioned to remain on the forefront of the emerging market for MRI-guided minimally invasive procedures.
Risks Related to Our Business
We are subject to a number of risks of which you should be aware before you decide to buy our common stock. These risks are discussed more fully in the “Risk Factors” section of this prospectus beginning on page 7 and should be read in their entirety. In general, we face risks associated with the following:
| • | convincing physicians and hospitals to use our ClearPoint system and achieving market acceptance for our marketed products; |
| • | our limited commercialization history; |
| • | the net losses that we have incurred in each year since our inception and expect to continue as we develop our business; |
| • | there is no guarantee that we will achieve the milestones under our agreements with Boston Scientific or be entitled to the milestone payments, and, if some of the milestones relating to neurological applications are not met by December 31, 2012, we will be required to repay to Boston Scientific amounts specified in the related development agreement; |
| • | obtaining FDA or other regulatory approvals or clearances of our product candidates; |
| • | any failure to comply with rigorous FDA and other government regulations; and |
| • | securing and maintaining patent or other intellectual property protection covering our marketed products and product candidates. |
Recent Developments
No material development has occurred since the conclusion of our quarterly period ended March 31, 2010 with the exception of our receipt of FDA clearance to market our ClearPoint system, which is discussed elsewhere in this prospectus.
Corporate Information
We were incorporated in Delaware in 1998 under the name Surgi-Vision, Inc. On November 12, 2008, we changed our name to SurgiVision, Inc. We operate in only one business segment. Our principal executive office is located at One Commerce Square, Suite 2550, Memphis, TN 38103, and our telephone number is (901) 522-9300. Our principal operations are located in Irvine, California. Our website address is www.surgivision.com. We do not incorporate the information on our website into this prospectus, and you should not consider it part of this prospectus.
4
Summary of the Offering
| Common stock offered by us |
2,500,000 shares (or 2,875,000 shares if the underwriters exercise their over-allotment option in full). We are not registering any shares of common stock issuable upon conversion of any of our convertible securities or any shares of common stock held by our stockholders. |
| Common stock to be outstanding after the offering |
10,129,405 shares (or 10,504,405 shares, if the underwriters exercise their over allotment option in full) |
| Nasdaq Capital Market symbol |
SRGV |
| Use of proceeds |
We expect to use the net proceeds from this offering to fund our research and development activities, sales and marketing activities and for working capital and other general corporate purposes. |
The number of shares of common stock to be outstanding after this offering is based on the number of shares outstanding as of April 30, 2010 and excludes:
| • | 600,625 shares of common stock issuable upon exercise of options issued under our stock option plans, at a weighted average exercise price of $3.61 per share; |
| • | 66,652 shares of common stock issuable upon the exercise of an option not issued under our stock option plans, at an exercise price of $9.64 per share; |
| • | 410,542 shares of common stock issuable upon exercise of warrants, at a weighted average exercise price of $3.48 per share; |
| • | 458,630 shares of common stock issuable upon the conversion of $3,669,040 in principal amount of, and interest on, convertible promissory notes, at a conversion price of $8.00 per share; |
| • | 125,000 shares of our common stock that may be issued to the underwriters, upon exercise of warrants, at an exercise price of $12.50 per share, assuming an initial public offering price of $10.00 per share, which is the mid-point of the range listed on the cover of this prospectus; |
| • | 25,444 shares of common stock that may be issued pursuant to the placement agent warrant, at an exercise price of $8.00 per share, assuming an initial public offering price of $10.00 per share, which is the mid-point of the range listed on the cover of this prospectus; |
| • | 12,500 shares of common stock issuable upon the exercise of a warrant to be issued in connection with this offering with an exercise price equal to the initial public offering price; |
| • | 541,000 shares of common stock issuable upon the exercise of options to be issued in connection with this offering under our 2010 Incentive Compensation Plan each with an exercise price equal to the initial public offering price; |
| • | 30,000 shares of common stock to be issued in connection with this offering under our 2010 Incentive Compensation Plan assuming an initial public offering price of $10.00 per share, which is the mid-point of the range listed on the cover of this prospectus; and |
| • | 679,000 shares of common stock reserved for future issuance under our 2010 Incentive Compensation Plan. |
Except as otherwise noted, all information in this prospectus:
| • | assumes no exercise of the underwriters’ over-allotment option; and |
| • | gives effect to a 1-for-4 reverse stock split and conversion into common stock of all outstanding shares of our preferred stock and our 10% senior unsecured convertible notes, or the bridge notes. |
5
Summary Financial Information
The summary financial information below should be read in conjunction with “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and the financial statements, notes thereto and other financial information included elsewhere in this prospectus. The information presented as of and for the three months ended March 31, 2010 and for the three months ended March 31, 2009 is derived from unaudited financial statements and includes, in the opinion of management, all adjustments, consisting only of normal recurring accruals, necessary to present fairly the information for such periods. The summary financial information for the fiscal years ended December 31, 2009, 2008 and 2007 has been derived from our audited financial statements and the notes thereto included elsewhere in this prospectus.
| Three Months Ended March 31, |
Years Ended December 31, | |||||||||||||||||||
| 2010 | 2009 | 2009 | 2008 | 2007 | ||||||||||||||||
| Statement of Operations Data: |
||||||||||||||||||||
| Related party license revenue |
$ | 650,000 | $ | 650,000 | $ | 2,600,000 | $ | 1,950,000 | $ | — | ||||||||||
| Operating costs and expenses: |
||||||||||||||||||||
| Research and development costs |
1,747,395 | 1,501,555 | 6,067,617 | 4,258,492 | 2,098,672 | |||||||||||||||
| General and administrative expenses |
1,011,747 | 605,683 | 3,595,917 | 2,920,311 | 1,413,369 | |||||||||||||||
| Total operating expenses |
2,759,142 | 2,107,238 | 9,663,534 | 7,178,803 | 3,512,041 | |||||||||||||||
| Other (income) expense |
406,570 | (32,325 | ) | 46,276 | 200,982 | 185,096 | ||||||||||||||
| Income tax expense |
— | — | 49,250 | — | — | |||||||||||||||
| Net loss |
$ | (2,515,712 | ) | $ | (1,424,913 | ) | $ | (7,159,060 | ) | $ | (5,429,785 | ) | $ | (3,697,137 | ) | |||||
| Net loss per share (basic and diluted) |
$ | (0.49 | ) | $ | (0.27 | ) | $ | (1.34 | ) | $ | (1.04 | ) | $ | (0.74 | ) | |||||
| Weighted average shares outstanding (basic and diluted) |
5,129,280 | 5,368,444 | 5,336,633 | 5,245,081 | 5,024,515 | |||||||||||||||
The following table presents a summary of our balance sheet as of March 31, 2010:
| • | on an actual basis; |
| • | on a pro forma basis to reflect a 1-for-4 reverse stock split and the conversion into common stock of all outstanding shares of our preferred stock and the bridge notes; and |
| • | on a pro forma as adjusted basis to reflect the pro forma adjustments reflected above and the sale in this offering of 2,500,000 shares of common stock at an assumed initial public offering price of $10.00 per share, which is the mid-point of the range listed on the cover of this prospectus, after deducting the estimated underwriting discounts and commissions and estimated offering expenses payable by us. |
| As of March 31, 2010 | ||||||||||||
| Actual | Pro Forma | Pro Forma as Adjusted |
||||||||||
| Balance Sheet Data: |
||||||||||||
| Cash and cash equivalents |
$ | 3,548,719 | $ | 3,548,719 | $ | 24,873,644 | ||||||
| Deferred revenue |
8,546,374 | 8,546,374 | 8,546,374 | |||||||||
| Convertible notes, net of discounts of $1,877,444 |
5,693,556 | 2,436,730 | 2,436,730 | |||||||||
| Convertible preferred stock |
7,965,000 | — | — | |||||||||
| Common stock and additional paid-in capital (less treasury stock) |
25,178,772 | 37,214,772 | 58,539,697 | |||||||||
| Accumulated deficit |
(44,538,676 | ) | (45,352,850 | ) | (45,352,850 | ) | ||||||
| Total stockholders’ equity (deficit) |
(11,394,904 | ) | (8,138,078 | ) | 13,186,847 | |||||||
6
Any investment in our common stock involves a high degree of risk. You should consider carefully the risks and uncertainties described below and all information contained in this prospectus, before you decide whether to purchase our common stock. If any of the following risks or uncertainties actually occurs, our business, financial condition, results of operations and prospects would likely suffer, possibly materially. In addition, the trading price of our common stock could decline due to any of these risks or uncertainties, and you may lose part or all of your investment.
Risks Related to Our Business
We have incurred significant losses since our inception and anticipate that we may continue to incur significant losses. If we fail to generate significant revenue from sales of our marketed products, we may never achieve or sustain profitability.
As of March 31, 2010, we had an accumulated deficit of approximately $44,539,000. The accumulated deficit has resulted principally from costs incurred in our research and development efforts and general operating expenses. We have incurred significant losses in each year since our inception in 1998. Net losses were approximately $2,516,000 for the three months ended March 31, 2010, approximately $7,159,000 for the year ended December 31, 2009, approximately $5,430,000 for the year ended December 31, 2008, and approximately $3,697,000 for the year ended December 31, 2007. We may continue to incur significant operating losses as we continue to invest capital in the sales and marketing of our marketed products, development of our product candidates and our business generally. We also expect that our general and administrative expenses will increase due to additional operational and regulatory burdens associated with operating as a public company.
As a result of the numerous risks and uncertainties associated with developing medical devices, we are unable to predict the extent of any future losses or when we will become profitable, if at all. Our profitability will depend on revenues from the sale of our marketed products. While we recently began marketing of our ClearPoint system in the United States by directly contacting key physicians and hospitals, we may never achieve significant revenues. Therefore, we cannot provide any assurance that we will ever achieve profitability and, even if we achieve profitability, that we will be able to sustain or increase profitability on a quarterly or annual basis. Further, because of our limited commercialization history, we have limited insight into the trends that may emerge and affect our business. We may make errors in predicting and reacting to relevant business trends, which could harm our business and financial condition. Any failure to achieve and maintain profitability would continue to have an adverse effect on our stockholders’ equity and working capital and could result in a decline in our stock price or cause us to cease operations.
Although we have obtained regulatory clearance to market our ClearPoint system in the United States, it may not achieve market acceptance or be commercially successful.
We expect sales of our ClearPoint system will account for the vast majority of our revenues for at least the next several years. Although we received regulatory clearance for our ClearPoint system and we have begun marketing in the United States by directly contacting key physicians and hospitals, our marketed products may not gain market acceptance unless we convince physicians, hospitals and patients of the benefits of our marketed products. Moreover, even if physicians and hospitals understand the benefits of our marketed products, they still may elect not to use our ClearPoint system for a variety of reasons, including:
| • | the shift in location of the procedure from the operating room to the MRI suite; |
| • | the hospital’s ability and willingness to satisfy the increased demand for the MRI suite; |
| • | the cost to the hospital to purchase or otherwise use our marketed products; |
| • | the amount of reimbursement available from third-party payors; |
7
| • | the lack of supporting clinical data; and |
| • | the physician’s familiarity, and having achieved successful results, with other devices and approaches. |
We believe that the market for our ClearPoint system is fairly concentrated among a few hundred hospitals. If physicians and hospitals do not perceive our ClearPoint system as an attractive alternative to other products and procedures, we will not achieve significant market penetration or be able to generate significant revenues, if any. To the extent that our ClearPoint system is not commercially successful or is withdrawn from the market for any reason, our revenues will be adversely impacted and our business, operating results and financial condition will be harmed.
If we fail to obtain regulatory approval for our ClearPoint system in foreign jurisdictions, we will not be able to expand the commercialization of our marketed products abroad.
Currently, we market our ClearPoint system in the United States; however, we also intend to sell our ClearPoint system in the European Union. To market a product in the European Union, we must be entitled to affix a CE mark, an international symbol of adherence to quality assurance standards and compliance with applicable European Union medical device directives. A CE mark would enable us to market a product in all of the countries of the European Union, as well as in other countries, such as Switzerland and Israel, that have mutual recognition agreements with the European Union or have adopted the European Union’s regulatory standards. There can be no assurance that we will receive CE marking approval for our ClearPoint system. To sell our ClearPoint system in other foreign jurisdictions, we will have to obtain separate regulatory approvals from those foreign jurisdictions as well. The regulatory approval process varies among jurisdictions and can involve substantial additional testing. Clearance or approval by the FDA does not ensure clearance or approval by regulatory authorities in other jurisdictions, and clearance or approval by one foreign regulatory authority does not ensure clearance or approval by regulatory authorities in other foreign jurisdictions. The foreign regulatory approval process may include all of the risks associated with obtaining FDA clearance or approval in addition to other risks. In addition, the time required to obtain foreign clearance or approval may differ from that required to obtain FDA clearance or approval and we may not obtain foreign regulatory clearances or approvals on a timely basis, if at all. We may not be able to file for regulatory clearance or approval and may not receive necessary clearance or approval to commercialize our ClearPoint system in any foreign market, either of which would preclude sale of our ClearPoint system in foreign jurisdictions.
We intend to apply for CE marking approval for sale of our ClearPoint system during 2010, and we have engaged KEMA as the Notified Body for our CE marking approval process. A Notified Body is a private commercial entity that is designated by the national government of a European Union member state as being competent to make independent judgments about whether a device complies with applicable regulatory requirements. The exact regulatory pathway for CE marking approval for our ClearPoint system will be the subject of discussions that we have with KEMA. At this time, we are unable to accurately predict when, if ever, CE marking for our ClearPoint system will be obtained, whether clinical trials will be required as part of the CE marking approval process or the regulatory requirements to which we would be subject after approval.
If hospitals and physicians are unable to obtain adequate coverage and reimbursement from third-party payors for procedures utilizing our ClearPoint system, our revenues and prospects for profitability will suffer.
We anticipate that our ClearPoint system components will be purchased primarily by hospitals, which bill various third-party payors, including governmental healthcare programs, such as Medicare, and private insurance plans, for procedures in which our marketed products will be used. Reimbursement is a significant factor considered by hospitals in determining whether to acquire new medical devices such as our marketed products. Therefore, our ability to successfully commercialize our ClearPoint system depends significantly on the availability of coverage and reimbursement from these third-party payors.
Medicare pays hospitals a prospectively determined amount for inpatient operating costs. The prospective payment for a patient’s stay is determined by the patient’s condition and other patient data and procedures
8
performed during the inpatient stay using a classification system known as Medical Severity Diagnosis Related Groups, or MS-DRGs. Medicare pays a fixed amount to the hospital based on the MS-DRG into which the patient’s stay is assigned, regardless of the actual cost to the hospital of furnishing the procedures, items and services provided. Therefore, a hospital must absorb the cost of our marketed products as part of the payment it receives for the procedure in which the product is used. In addition, physicians that perform procedures in hospitals are paid a set amount by Medicare for performing such services under the Medicare physician fee schedule. Medicare payment rates for both systems are established annually.
At this time, we do not know if hospitals will consider third-party reimbursement levels adequate to cover the cost of our marketed products. Furthermore, we do not know if physicians will consider third-party reimbursement levels adequate to compensate them for performing the procedures in which our marketed products are used. Failure by hospitals and physicians to receive an amount that they consider to be adequate reimbursement for procedures in which our marketed products are used will deter them from purchasing or using our marketed products and limit our sales growth.
One result of the current Medicare payment system, which is also utilized by most non-governmental third-party payors, is that a patient’s treating physician orders a particular service and the hospital (or other facility in which the procedure is performed) bears the cost of delivery of the service. Hospitals have limited ability to align their financial interests with those of the treating physician because Medicare law generally prohibits hospitals from paying physicians to assist in controlling the costs of hospital services, including paying physicians to limit or reduce services to Medicare beneficiaries even if such services are medically unnecessary. As a result, hospitals have traditionally stocked supplies and products requested by physicians and have had limited ability to restrict physician choice of products and services.
The Patient Protection and Affordable Care Act enacted on March 23, 2010, as amended by the Health Care and Education Reconciliation Act of 2010 enacted on March 30, 2010, or, together, the Health Care Reform Law, includes a number of provisions that will likely result in more coordination between hospitals and physicians resulting in the alignment of financial incentives between hospitals and physicians to control hospital costs. Most significantly, the Health Care Reform Law provides for the establishment of a Medicare shared savings program whereby Medicare will share certain savings realized in the delivery of services to Medicare beneficiaries with accountable care organizations, which may be organized through various different legal structures between hospitals and physicians. We expect that the overall result of such increased coordination will be voluntary reductions in the array of choices currently available to physicians with respect to diagnostic services, medical supplies and equipment. Such a reduction in physician choices may also result in hospitals reducing the overall number of vendors from which they purchase supplies, equipment and products. The Health Care Reform Law may make it more difficult for us to become and remain an approved vendor, which could have an adverse effect on our financial results and business.
If there are changes in coverage or reimbursement from third-party payors, our revenues and prospects for profitability will suffer.
In the United States, we believe that existing billing codes apply to procedures using our ClearPoint system. Reimbursement levels for procedures using our ClearPoint system or any product that we may market in the future could be decreased or eliminated as a result of future legislation, regulation or reimbursement policies of third-party payors. Any such decrease or elimination would adversely affect the demand for our ClearPoint system or any product that we may market in the future and our ability to sell our products on a profitable basis. For example, on July 30, 2008, Centers for Medicare and Medicaid Services, or CMS, the federal agency that administers the Medicare Program, released a list of potential topics for national coverage determinations. This list included ablation for atrial fibrillation and specifically asked whether the evidence was adequate to demonstrate health benefits in patients who receive the procedure. On October 21, 2009, the Medicare Evidence Development and Coverage Advisory Committee held a meeting on the adequacy of the available evidence for catheter ablation for the treatment of atrial fibrillation. Although CMS has not formally opened a national coverage analysis on this topic, the agency has shown that it is interested in the clinical evidence of atrial
9
fibrillation treatments and any national coverage decisions it makes could have a material effect on the ClearTrace system and our potential business in this area. Furthermore, if procedures using our ClearPoint system gain market acceptance and the number of these procedures increases, CMS, as well as other public or private payors, may establish new billing codes for those procedures that provide for a lower reimbursement amount than traditional approaches, which would adversely affect our financial results and business.
Among other things, the Health Care Reform Law will ultimately increase the overall pool of persons with access to health insurance in the United States. Although such an increase in covered lives should ultimately benefit hospitals, the Health Care Reform Law also includes a number of cuts in Medicare reimbursement to hospitals that may take effect prior to the time hospitals’ realize the financial benefit of a larger pool of insured persons. Such cuts in Medicare reimbursement could adversely impact the operations and finances of hospitals, reducing their ability to purchase medical devices such as our ClearPoint system. Further, the fact that the Health Care Reform Law did not address pending reductions of Medicare physician payment rates under the sustainable growth rate formula could result in an overall reduction of physicians willing to participate in Medicare. Either of these events could adversely affect demand for our ClearPoint system, our business and our financial results.
If third-party payors deny coverage or reimbursement for procedures using our ClearPoint system, our revenues and prospects for profitability will suffer.
Notwithstanding its regulatory clearance in the United States, third-party payors may deny coverage or reimbursement if the payor determines that the use of our ClearPoint system is unnecessary, inappropriate, experimental, not cost-effective, or is used for a non-approved indication. In addition, no uniform policy of coverage and reimbursement for medical technology exists among third-party payors. Therefore, coverage and reimbursement for medical technology can differ significantly from payor to payor. Any denial of coverage or reimbursement for procedures using our ClearPoint system could have an adverse effect on our business, financial results and prospects for profitability.
If we are unable to expand our sales, marketing and distribution capabilities or enter into agreements with third parties to market, sell or distribute our ClearPoint system, we may be unable to generate material product revenues.
We have limited experience in the sales and marketing of medical devices. Currently, our sales and marketing efforts for our ClearPoint system are being coordinated primarily by our Vice President, Sales, our Vice President, Product Management and our two Clinical Engineering Managers. In order to successfully commercialize our ClearPoint system, we will need to expand our present sales and marketing capabilities, which could prove to be time-consuming and expensive. If we are unable to expand these capabilities, we will need to contract with third parties to help us market and sell our ClearPoint system. Likewise, if our current distribution capabilities are unable to satisfy customer demand for our ClearPoint system, we will need to contract with third parties to help us perform that function. To the extent that we enter into arrangements with third parties to perform sales and marketing or distribution services, our product revenues are likely to be lower than if we market, sell and distribute our ClearPoint system ourselves.
Our reliance on single-source suppliers could harm our ability to meet demand for our ClearPoint system in a timely manner or within budget.
Many of the components and component assemblies of our ClearPoint system are currently provided to us by single-source suppliers. We generally purchase components and component assemblies through purchase orders rather than long-term supply agreements and generally do not maintain large volumes of inventory. While alternative suppliers exist and have been identified, the disruption or termination of the supply of components and component assemblies could cause a significant increase in the cost of these components, which could affect our operating results. Our dependence on a limited number of third-party suppliers and the challenges we may face in obtaining adequate supplies involve several risks, including limited control over pricing, availability, quality and delivery schedules. A disruption or termination in the supply of components could also result in our inability to meet demand for our ClearPoint system, which could harm our ability to generate revenues, lead to
10
customer dissatisfaction and damage our reputation. Furthermore, if we are required to change the supplier of a key component or component assembly of our ClearPoint system, we may be required to verify that the new supplier maintains facilities and procedures that comply with quality standards and with all applicable regulations and guidelines. The delays associated with the verification of a new supplier could delay our ability to manufacture our ClearPoint system in a timely manner or within budget.
The Health Care Reform Law and other payment and policy changes may have a material adverse effect on us.
In addition to the reimbursement changes discussed above, the Health Care Reform Law will also impose a 2.3% excise tax on the sale of any taxable human medical device after December 31, 2012, subject to certain exclusions, by the manufacturer, producer or importer of such devices. Further, the Health Care Reform Law encourages hospitals and physicians to work collaboratively through shared savings programs, such as accountable care organizations, which may ultimately result in the reduction of medical device acquisitions and the consolidation of medical device suppliers used by hospitals. While passage of the Health Care Reform Law may ultimately expand the pool of potential end-users of our ClearPoint system, the above-discussed changes could adversely affect our financial results and business.
Further, with the increase in demand for healthcare services, we expect both a strain on the capacity of the healthcare system and more proposals by legislators, regulators and third-party payors to keep healthcare costs down. Certain proposals, if passed, could impose limitations on the prices we will be able to charge for our ClearPoint system, or the amounts of reimbursement available from governmental agencies or third-party payors. These limitations could have a material adverse effect on our financial position and results of operations.
Various healthcare reform proposals have also emerged at the state level. We cannot predict what healthcare initiatives, if any, will be implemented at the federal or state level, or the effect any future legislation or regulation will have on us. However, an expansion in government’s role in the United States healthcare industry may lower reimbursements for our ClearPoint system, reduce medical procedure volumes and adversely affect our business, possibly materially.
Our future success depends on our ability to obtain regulatory clearances or approvals for our current product candidates. We cannot be certain that we will be able to do so in a timely fashion, or at all.
We do not have the necessary regulatory clearances or approvals to market the ClearTrace system in the United States or in any foreign market. In the United States, without FDA clearances or approvals, we cannot market a new medical device, or a new use of, or claim for, or significant modification to, an existing product, unless an exemption applies. To obtain FDA clearance or approval, we must first receive either premarket clearance under Section 510(k) of the federal Food, Drug, and Cosmetic Act or approval of a premarket approval application, or PMA, from the FDA.
In the 510(k) clearance process, the FDA must determine that a proposed device is “substantially equivalent” to a device legally on the market, known as a “predicate” device, with respect to intended use, technology, safety and effectiveness, in order to clear the proposed device for marketing. Clinical data is sometimes required to support substantial equivalence. The 510(k) clearance process generally takes three to twelve months from submission, but can take significantly longer.
The process of obtaining PMA approval is much more costly and uncertain than the 510(k) clearance process. The PMA approval process can be lengthy and expensive and requires an applicant to demonstrate the safety and effectiveness of the device based, in part, on data obtained in clinical trials. The PMA process generally takes one to three years, or even longer, from the time the PMA application is submitted to the FDA until an approval is obtained.
11
Outside the United States, the regulatory approval process varies among jurisdictions and can involve substantial additional testing. Clearance or approval by the FDA does not ensure clearance or approval by regulatory authorities in other jurisdictions, and clearance or approval by one foreign regulatory authority does not ensure clearance or approval by regulatory authorities in other foreign jurisdictions. The foreign regulatory approval process may include all of the risks associated with obtaining FDA clearance or approval in addition to other risks. In addition, the time required to obtain foreign clearance or approval may differ from that required to obtain FDA clearance or approval and we may not obtain foreign regulatory clearances or approvals on a timely basis, if at all. We may not be able to file for regulatory clearance or approval and may not receive necessary clearance or approval to commercialize our product candidates in any foreign market, either of which would preclude sale of our product candidates in foreign jurisdictions.
To market a product in the European Union, we must be entitled to affix a CE mark, an international symbol of adherence to quality assurance standards and compliance with applicable European medical device directives. CE marking approval would enable us to market a product in all of the countries of the European Union, as well as in other countries, such as Switzerland and Israel, that have mutual recognition agreements with the European Union or have adopted the European Union’s regulatory standards.
The regulatory status of our current product candidates is as follows:
| • | ClearTrace System. We are still in the early stages of the development of the ClearTrace system and have not made any regulatory filings with the FDA or any foreign regulatory authority with respect to that system. We anticipate that the initial market for the ClearTrace system will be the European Union and we plan to seek CE marking approval for the ClearTrace system, although there can be no assurance that we will receive CE marking approval. The ClearTrace system consists of several components, including an ablation catheter. Whether as part of the regulatory process in the United States or the CE marking approval process in the European Union, we expect to conduct a clinical trial regarding the safety and effectiveness of our ablation catheter, and we expect to commence enrollment in such a clinical trial in the second half of 2011. The FDA has determined that ablation catheters specifically indicated to treat atrial fibrillation require the submission of a PMA. Therefore, in the United States, we will be required to pursue the PMA process in order to specifically indicate our ablation catheter for the treatment of atrial fibrillation. |
| • | SafeLead Development Program. We are still in the early stages of the SafeLead Development Program. Boston Scientific is responsible for making any regulatory filings with respect to its products that incorporate our MRI-safety technologies. Boston Scientific will control the timing and manner of any regulatory filing, and will be responsible for the costs associated with any regulatory filing. We do not anticipate that we will be able to influence the process or timing in any meaningful way. No regulatory filings have been made to date with the FDA or any foreign regulatory authority. |
The FDA or any applicable foreign authority may not act favorably or quickly in its review of any regulatory submission that we may file or that Boston Scientific may file in connection with the SafeLead Development Program. Additionally, we or Boston Scientific may encounter significant difficulties and costs in obtaining clearances or approvals. If we or Boston Scientific, as the case may be, are unable to obtain regulatory clearances or approvals for our product candidates, or otherwise experience delays in obtaining regulatory clearances or approvals, the commercialization of our product candidates will be delayed or prevented, which will adversely affect our ability to generate revenues. Such delay may also result in the loss of potential competitive advantages that might otherwise be attained by bringing products to market earlier than competitors. Any of these contingencies could adversely affect our business. Even if cleared or approved, our product candidates may not be cleared or approved for the indications that are necessary or desirable for successful commercialization.
12
To the extent we seek a new indication for use of, or new claims for, our ClearPoint system, the FDA may not grant 510(k) clearance or PMA approval of such new use or claims, which may affect our ability to grow our business.
We received 510(k) clearance to market our ClearPoint system for use in general neurological interventional procedures. In the future, we may seek to obtain additional, more specific indications for use of our ClearPoint system beyond the general neurological intervention claim. Some of these expanded claims may require FDA 501(k) clearance. Other claims may require FDA approval of a PMA. Moreover, some specific ClearPoint system claims that we may seek may require clinical trials to support regulatory clearance or approval, and we may not successfully complete or have the funds to initiate these clinical trials. The FDA may not clear or approve these future claims or future generations of our ClearPoint system for the indications that are necessary or desirable for successful commercialization. Indeed, the FDA may refuse our requests for 510(k) clearance or PMA approval. Failure to receive clearance or approval for additional claims for our ClearPoint system would have an adverse effect on our ability to expand our business.
Clinical trials necessary to support 510(k) clearance or PMA approval for the ClearTrace system or any new indications for use for our ClearPoint system will be expensive and may require the enrollment of large numbers of suitable patients, who may be difficult to identify and recruit. Delays or failures in our clinical trials will prevent us from commercializing any modified or new product candidates and will adversely affect our business, operating results and prospects.
Initiating and completing clinical trials necessary to support a PMA for the ClearTrace system or any other product candidates that we may develop, or additional safety and efficacy data that the FDA may require for 510(k) clearance or PMA approval for any new specific indications of our ClearPoint system that we may seek, will be time consuming and expensive with an uncertain outcome. Moreover, the results of early clinical trials are not necessarily predictive of future results, and any product candidate we advance into clinical trials may not have favorable results in later clinical trials.
Conducting successful clinical trials may require the enrollment of large numbers of patients, and suitable patients may be difficult to identify and recruit. Patient enrollment in clinical trials and completion of patient participation and follow-up depends on many factors, including the size of the patient population, the nature of the trial protocol, the attractiveness of, or the discomforts and risks associated with, the treatments received by enrolled subjects, the availability of appropriate clinical trial investigators and support staff, the proximity to clinical sites of patients that are able to comply with the eligibility and exclusion criteria for participation in the clinical trial, and patient compliance. For example, patients may be discouraged from enrolling in our clinical trials if the trial protocol requires them to undergo extensive post-treatment procedures or follow-up to assess the safety and effectiveness of our product candidates or if they determine that the treatments received under the trial protocols are not attractive or involve unacceptable risks or discomforts. In addition, patients participating in clinical trials may die before completion of the trial or suffer adverse medical events unrelated to our product candidates.
Development of sufficient and appropriate clinical protocols to demonstrate safety and efficacy will be required and we may not adequately develop such protocols to support clearance or approval. Further, the FDA may require us to submit data on a greater number of patients than we originally anticipated and/or for a longer follow-up period or change the data collection requirements or data analysis applicable to our clinical trials. Delays in patient enrollment or failure of patients to continue to participate in a clinical trial may cause an increase in costs and delays in the approval and attempted commercialization of our product candidates or result in the failure of the clinical trial. Such increased costs and delays or failures could adversely affect our business, operating results and prospects.
13
If the third parties on which we may need to rely to conduct any clinical trials and to assist us with pre-clinical development do not perform as contractually required or expected, we may not be able to obtain regulatory clearance or approval for the ClearTrace system or any additional claims that we may seek for our ClearPoint system.
We do not have the independent ability to conduct pre-clinical and clinical trials for our marketed products or our product candidates. To the extent that we will need to conduct such trials, we will need to rely on third parties, such as contract research organizations, medical institutions, clinical investigators and contract laboratories, to conduct such trials. If these third parties do not successfully carry out their contractual duties or regulatory obligations or meet expected deadlines, if these third parties need to be replaced, or if the quality or accuracy of the data they obtain is compromised due to the failure to adhere to our clinical protocols or regulatory requirements or for other reasons, our pre-clinical development activities or clinical trials may be extended, delayed, suspended or terminated, and we may not be able to obtain regulatory clearance or approval for a product candidate or additional claims we may seek for our marketed products on a timely basis, if at all. As such, our business, operating results and prospects may be adversely affected. Furthermore, our third-party clinical trial investigators may be delayed in conducting our clinical trials for reasons outside of their control.
The results of our clinical trials may not support our product candidate claims or any additional claims we may seek for our marketed products and may result in the discovery of adverse side effects.
Even if any clinical trial that we need to undertake is completed as planned, we cannot be certain that its results will support our product candidate claims or any new indications that we may seek for our marketed products or that the FDA or foreign authorities will agree with our conclusions regarding the results of those trials. The clinical trial process may fail to demonstrate that our marketed products or a product candidate is safe and effective for the proposed indicated use, which could cause us to stop seeking additional clearances or approvals for our ClearPoint system, abandon a product candidate and may delay development of others. Any delay or termination of our clinical trials will delay the filing of our regulatory submissions and, ultimately, our ability to commercialize a product candidate. It is also possible that patients enrolled in clinical trials will experience adverse side effects that are not currently part of the product candidate’s profile.
The markets for medical devices, such as our ClearPoint system and our product candidates, are highly competitive and we may not be able to compete effectively against the larger, well-established companies in our markets or emerging and small innovative companies that may seek to obtain or increase their share of the market.
We will face competition from products and techniques already in existence in the marketplace. The markets for our ClearPoint system and our product candidates are intensely competitive, and many of our competitors are much larger and have substantially more financial and human resources than we do. Many have long histories and strong reputations within the industry, and a relatively small number of companies dominate these markets. Examples of such large, well-known companies include Biosense Webster Inc., a division of Johnson & Johnson, Medtronic, Inc. and St. Jude Medical Inc.
These companies enjoy significant competitive advantages over us, including:
| • | broad product offerings, which address the needs of physicians and hospitals in a wide range of procedures; |
| • | greater experience in, and resources for, launching, marketing, distributing and selling products, including strong sales forces and established distribution networks; |
| • | existing relationships with physicians and hospitals; |
| • | more extensive intellectual property portfolios and resources for patent protection; |
| • | greater financial and other resources for product research and development; |
14
| • | greater experience in obtaining and maintaining FDA and other regulatory clearances or approvals for products and product enhancements; |
| • | established manufacturing operations and contract manufacturing relationships; and |
| • | significantly greater name recognition and more recognizable trademarks. |
We may not succeed in overcoming the competitive advantages of these large and established companies. Smaller or early-stage companies may also prove to be significant competitors, particularly through collaborative arrangements with large and established companies. These companies may introduce products that compete effectively against our products in terms of performance, price or both.
We could become subject to product liability claims that could be expensive, divert management’s attention and harm our business.
Our business exposes us to potential product liability risks that are inherent in the manufacturing, marketing and sale of medical devices. We may be held liable if our products cause injury or death or are found otherwise unsuitable or defective during usage. Our ClearPoint system and the ClearTrace system incorporate mechanical and electrical parts, complex computer software and other sophisticated components, any of which can have defective or inferior parts or contain defects, errors or failures. Complex computer software is particularly vulnerable to errors and failures, especially when first introduced.
Because our ClearPoint system and the ClearTrace system are designed to be used to perform complex surgical procedures, defects could result in a number of complications, some of which could be serious and could harm or kill patients. The adverse publicity resulting from any of these events could cause physicians or hospitals to review and potentially terminate their relationships with us.
The medical device industry has historically been subject to extensive litigation over product liability claims. A product liability claim, regardless of its merit or eventual outcome, could result in significant legal defense costs. Although we maintain product liability insurance, the coverage is subject to deductibles and limitations, and may not be adequate to cover future claims. Additionally, we may be unable to maintain our existing product liability insurance in the future at satisfactory rates or in adequate amounts. A product liability claim, regardless of its merit or eventual outcome could result in:
| • | decreased demand for our marketed products; |
| • | injury to our reputation; |
| • | diversion of management’s attention; |
| • | significant costs of related litigation; |
| • | payment of substantial monetary awards by us; |
| • | product recalls or market withdrawals; |
| • | a change in the design, manufacturing process or the indications for which our marketed products may be used; |
| • | loss of revenue; and |
| • | an inability to commercialize product candidates. |
We may not realize the anticipated benefits from our collaborative agreement with Siemens regarding the ClearTrace system.
We have entered into a co-development agreement with Siemens to develop the hardware and MRI software necessary for the ClearTrace system. There can be no assurance that our co-development efforts will be successful or that we will complete development of the ClearTrace system hardware and MRI software. Under
15
our agreement, Siemens is responsible for developing the software for the ClearTrace system, and we are responsible for developing the catheters and other hardware, other than the MRI scanner and workstation. We are obligated to pay Siemens up to approximately $2,500,000 in milestone payments associated with Siemens’ successful development of the software in accordance with our specifications. We started making these payments in the second quarter of 2009 and will continue through the third quarter of 2011. Once the software for the ClearTrace system is commercially available, Siemens is obligated to pay us a fixed amount for each software license sold by Siemens until we recoup our investment in the software. However, if Siemens does not successfully commercialize the software, or if our agreement with Siemens is terminated, we may not recover our investment in the software.
We may not realize the anticipated benefits from our collaborative agreements with Boston Scientific regarding the SafeLead Development Program.
We entered into license and development agreements with Boston Scientific with respect to our MRI-safety technologies. We are working with Boston Scientific to incorporate our MRI-safety technologies into Boston Scientific’s implantable medical leads for cardiac and neurological applications. There is no assurance that our joint development efforts will be successful or that patents will issue on any patent applications we licensed to Boston Scientific, in which case we would not receive future milestone payments or royalties provided for under our agreements with Boston Scientific. Further, Boston Scientific has no obligation to include our licensed intellectual property in its product candidates. Even if Boston Scientific incorporates our licensed intellectual property into its product candidates, Boston Scientific may be unable to obtain regulatory clearance or approval or successfully commercialize the related products, in which case we would not receive royalties in the amounts that we currently anticipate.
We may be required to pay amounts to Boston Scientific under our development agreement in the neurological field if all of our development milestones under that agreement are not met by December 31, 2012.
Our development agreement with Boston Scientific in the neurological field requires specified milestones in the development of an MRI-safe implantable lead to be achieved by December 31, 2012. If the milestones are not achieved by that date, and this failure is not the result of Boston Scientific’s failure to reasonably cooperate with us in pursuing the milestones, we will be required to pay Boston Scientific a sum of money equal to all milestone payments previously paid to us by Boston Scientific under the development agreement, all development expense reimbursements previously paid to us by Boston Scientific under the development agreement, and all patent prosecution costs incurred by Boston Scientific with respect to the intellectual property licensed under the related license agreement. As of March 31, 2010, the potential obligation to Boston Scientific was approximately $750,000, plus costs incurred by Boston Scientific in prosecuting the licensed intellectual property. Our potential payment obligation to Boston Scientific under the neuro development agreement does not apply to any amounts we receive under our agreements in the cardiac field, including the $13,000,000 of upfront licensing fees and any development milestone payments. Our agreements with Boston Scientific in the cardiac field do not impose a payment obligation on us for failure to achieve development milestones.
Boston Scientific has the right to terminate our development agreement for implantable cardiac leads under specified circumstances.
Boston Scientific has the one-time option, within 60 days after successful completion of the first lead feasibility study for cardiac applications, to cease further development work and to terminate the development agreement. If Boston Scientific elects to exercise its termination option under the development agreement, the license we granted to Boston Scientific in that field of use will automatically become non-exclusive with respect to some intellectual property, other intellectual property will be removed from the scope of the license and all rights will revert to us, and Boston Scientific will not be obligated to pay us any future royalties based on sales of its products containing our intellectual property that remains subject to the non-exclusive license.
16
Risks Related to our Need for Financing
We may not be able to continue operations as a going concern and our stockholders may lose their entire investment in us.
At March 31, 2010 and December 31, 2009, we had cash and cash equivalents of approximately $3,549,000 and $2,569,000, respectively, and stockholders’ deficit of approximately $11,395,000 and $9,888,000, respectively. In addition, we had a net loss for the three months ended March 31, 2010 of approximately $2,516,000 and a net loss for the year ended December 31, 2009 of approximately $7,159,000.
As discussed in note 3 to our financial statements included elsewhere in this prospectus, our cumulative net loss since inception and the net losses we incurred in 2009, 2008 and 2007 raise substantial doubt that we will be able to continue operations as a going concern. Our independent auditors included an explanatory paragraph regarding the uncertainty of whether we will be able to continue operations as a going concern in their report on our financial statements for the year ended December 31, 2009. Our ability to continue as a going concern is dependent upon us generating cash flow sufficient to fund operations and reducing operating expenses. Our business plans may not be successful in addressing these issues. If we cannot continue as a going concern, our stockholders may lose their entire investment in us.
We may need additional funding to continue to commercialize our marketed products and to bring our product candidates to market and we may not be able to raise capital when needed, which would force us to delay, reduce or eliminate our product development programs or commercialization efforts.
We will require substantial future capital in order to continue to establish effective marketing and sales capabilities for our ClearPoint system and conduct the research and development and regulatory clearance and approval activities necessary to bring our product candidates to market. If we are unable to generate revenue, we do not expect our existing capital resources and the net proceeds from this offering to be sufficient to enable us to fund the completion of the development and commercialization of all of our product candidates. Excluding cash generated from sales of our marketed products, we believe that the net proceeds from this offering, our existing cash resources and interest on these funds will be sufficient to meet our projected operating requirements through the end of 2011. However, our operating plans may change, and we may need additional funds sooner than anticipated to meet our operational needs and capital requirements for product development, clinical trials, regulatory clearances and approvals, and product commercialization.
Additional funds may not be available when we need them on terms that are acceptable to us, or at all. If adequate funds are not available on a timely basis, we may terminate or delay the development of one or more of our product candidates, or delay establishment of sales and marketing capabilities or other activities necessary to commercialize our product candidates successfully.
Our future funding requirements will depend on many factors, including:
| • | the scope, rate of progress and cost of our research and development activities; |
| • | the achievement of milestone events under, and other matters related to, our agreements with Boston Scientific and Siemens; |
| • | the terms and timing of any future collaborative, licensing or other arrangements that we may establish; |
| • | the cost and timing of clinical trials; |
| • | the cost and timing of regulatory filings, clearances and approvals; |
| • | the cost and timing of establishing sales, marketing and distribution capabilities and other corporate infrastructure; |
| • | the cost of establishing product inventories; |
17
| • | the effect of competing technological and market developments; and |
| • | the cost of filing, prosecuting, defending and enforcing any patent claims and other intellectual property rights. |
Raising additional capital by issuing securities or through collaborative or licensing arrangements may cause dilution to existing stockholders, restrict our operations or require us to relinquish proprietary rights.
To the extent that we raise additional capital through the sale of equity or convertible debt securities, your ownership interest will be diluted, and the terms may include liquidation or other preferences that adversely affect your rights as a stockholder. Debt financing, if available, may involve agreements that include covenants limiting or restricting our ability to take specific actions such as incurring additional debt, making capital expenditures or declaring dividends. If we raise additional funds through collaboration or licensing arrangements with third parties, we may have to relinquish valuable rights to our technologies or products or grant licenses on terms that are not favorable to us. Any of these events could adversely affect our ability to achieve our product development and commercialization goals and have a material adverse effect on our business, financial condition and results of operations.
If we are unable to generate sufficient revenues from product sales, we may not have the funds to make required payments on certain of our convertible promissory notes.
During 2009, Boston Scientific loaned us $3,500,000 pursuant to the terms of three convertible promissory notes. Interest on the loans accrues at 10% per annum and compounds annually. The Boston Scientific loans are secured by a first priority security interest in all of our assets. Each loan matures on the second anniversary of the date on which the funds were advanced, which will occur in October, November and December of 2011, respectively. On June 16, 2010, we received 510(k) clearance from the FDA to market our ClearPoint system in the United States. While we recently began marketing in the United States by directly contacting key physicians and hospitals, we have not generated revenues from product sales. If we are unable to generate sufficient product revenues, we may not have the funds necessary to make the required interest and principal payments on the Boston Scientific loans when due. If we do not have sufficient funds to make these payments, we will seek an alternative source of funds, which may include sales of our assets or equity securities or additional borrowings. We cannot assure you that an alternative source of funds would be available on terms acceptable to us, or at all, which would have a material adverse effect on our financial condition.
Risks Related to our Intellectual Property
We are currently subject to intellectual property litigation. We may spend substantial funds to defend ourselves in the litigation and, if we are unsuccessful in our defense, we may be required to change our name and incur substantial additional costs, all of which could harm our operating results.
On April 22, 2010, SurgiVision Consultants, Inc. and Guy M. Kezirian filed a lawsuit against us in the United States District Court, Central District of California, alleging trademark infringement, unfair competition, trademark dilution and violation of the Anti-Cybersquatting Protection Act, all relating to our use of our SURGI- VISION and SURGIVISION trademarks and our www.surgivision.com domain name. The plaintiffs are seeking unspecified monetary damages and injunctive relief. This action is at a very preliminary stage. We believe that we have strong defenses to the allegations, and we intend to vigorously defend ourselves in the lawsuit to protect our rights. However, intellectual property litigation is inherently time consuming, expensive and unpredictable. If we are unsuccessful in the litigation, we may be required to change our name as well as pay monetary damages. If we are required to change our name, we may incur substantial costs and suffer from a loss of name recognition, which could harm our business, operating results and financial condition.
If we, or the third parties from whom we license intellectual property, are unable to secure and maintain patent or other intellectual property protection for the intellectual property covering our marketed products or our product candidates, our ability to compete will be harmed.
Our commercial success depends, in part, on obtaining patent and other intellectual property protection for the technologies contained in our marketed products and product candidates. The patent positions of medical
18
device companies, including ours, can be highly uncertain and involve complex and evolving legal and factual questions. Our patent position is uncertain and complex, in part, because of our dependence on intellectual property that we license from others. If we, or the third parties from whom we license intellectual property, fail to obtain adequate patent or other intellectual property protection for intellectual property covering our marketed products or product candidates, or if any protection is reduced or eliminated, others could use the intellectual property covering our marketed products or product candidates, resulting in harm to our competitive business position. In addition, patent and other intellectual property protection may not provide us with a competitive advantage against competitors that devise ways of making competitive products without infringing any patents that we own or have rights to.
As of April 30, 2010, our portfolio included eight wholly-owned issued United States patents (including one design patent), 25 wholly-owned pending United States patent applications (including five provisional applications), four co-owned issued United States patents, nine co-owned pending United States patent applications, one wholly-owned issued foreign patent, 38 wholly-owned pending foreign patent applications (including seven Patent Cooperation Treaty applications), one co-owned issued foreign patent and 22 co-owned pending foreign patent applications (including one Patent Cooperation Treaty application). In addition, as of April 30, 2010, we had licensed rights to 11 United States and 15 foreign third-party issued patents, and we had licensed rights to nine United States and 12 foreign third-party pending patent applications. United States patents and patent applications may be subject to interference proceedings and United States patents may be subject to reissue and reexamination proceedings in the United States Patent and Trademark Office. Foreign patents may
be subject to opposition or comparable proceedings in the corresponding foreign patent offices. Any of these proceedings could result in either loss of the patent or denial of the patent application, or loss or reduction in the scope of one or more of the claims of the patent or patent application. Changes in either patent laws or in interpretations of patent laws may also diminish the value of our intellectual property or narrow the scope of our protection. Interference, reexamination and opposition proceedings may be costly and time consuming, and we, or the third parties from whom we license intellectual property, may be unsuccessful in such proceedings. Thus, any patents that we own or license may provide limited or no protection against competitors. In addition, our pending patent applications and those we may file in the future may not result in patents being issued or may have claims that do not cover our marketed products or product candidates. Even if any of our pending or future patent applications are issued, they may not provide us with adequate protection or any competitive advantages. Our ability to develop additional patentable technology is also uncertain.
Non-payment or delay in payment of patent fees or annuities, whether intentional or unintentional, may also result in the loss of patents or patent rights important to our business. Many countries, including certain countries in Europe, have compulsory licensing laws under which a patent owner may be compelled to grant licenses to third parties. In addition, many countries limit the enforceability of patents against third parties, including government agencies or government contractors. In these countries, the patent owner may have limited remedies, which could materially diminish the value of the patent. In addition, the laws of some foreign countries do not protect intellectual property rights to the same extent as do the laws of the United States, particularly in the field of medical devices and procedures.
Others may assert that our marketed products or product candidates infringe their intellectual property rights, which may cause us to engage in costly disputes and, if we are not successful in defending ourselves, could also cause us to pay substantial damages and prohibit us from selling our marketed products.
There may be United States and foreign patents issued to third parties that relate to our business, including MRI-guided intervention systems and the components and methods and processes related to these systems. Some of these patents may be broad enough to cover one or more aspects of our present technologies and/or may cover aspects of our future technologies. We do not know whether any of these patents, if asserted, would be held valid, enforceable and infringed.
We cannot assure that a court or administrative body would agree with any arguments or defenses we may have concerning invalidity, unenforceability or non-infringement of any third-party patent. In addition, other
19
parties may have filed or may in the future file patent applications for products that are similar or identical to ours. We cannot assure that any patents issuing from applications filed by a third party will not cover our marketed products or product candidates or will not have priority over our patents or patent applications.
The medical device industry has been characterized by extensive litigation and administrative proceedings regarding patents and other intellectual property rights, and companies have employed such actions to gain a competitive advantage. If third parties assert infringement or other intellectual property claims against us, our technical and management personnel will experience a significant diversion of time and effort and we will incur large expenses defending our company. If third parties in any patent action are successful, our patent portfolio may be damaged, we may have to pay substantial damages and we may be required to stop selling our marketed products or obtain a license which, if available at all, may require us to pay substantial royalties. We cannot be certain that we will have the financial resources or the substantive arguments to defend our marketed products or product candidates from infringement or our patents from claims of invalidity or unenforceability, or to defend our marketed products or product candidates against allegations of infringement of third-party patents. In addition, any public announcements related to litigation or administrative proceedings initiated by us, or initiated or threatened against us, could cause our stock price to decline.
If we lose access to critical third-party software that is integrated into our ClearPoint system software, our costs could increase and sales of our ClearPoint system would be delayed, potentially hurting our competitive position.
We license software from a third party that is integrated into the software component of our ClearPoint system. Our license continues through July 2015. If we are unable to continue to license this third-party software, we would not be able to continue to commercialize our ClearPoint system until equivalent software could be identified, licensed or developed, and integrated into the software component of our ClearPoint system. These delays, if they occur, could harm our business, operating results and financial condition.
We may be subject to damages resulting from claims that our employees or we have wrongfully used or disclosed alleged trade secrets or other proprietary information of their former employers.
Many of our employees were previously employed at universities or other medical device companies, including competitors or potential competitors. In the future, we could be subject to claims that these employees, or we, have inadvertently or otherwise used or disclosed trade secrets or other proprietary information of their former employers. Litigation may be necessary to defend against these claims. If we fail in defending against such claims, a court could order us to pay substantial damages and prohibit us from using technologies or features that are essential to our marketed products and product candidates, if such technologies or features are found to incorporate or be derived from the trade secrets or other proprietary information of the former employers. In addition, we may lose valuable intellectual property rights or personnel. A loss of key research personnel or their work product could hamper or prevent our ability to commercialize certain product candidates, which could severely harm our business. Even if we are successful in defending against these claims, such litigation could result in substantial costs and be a distraction to our employees and management.
If the combination of patents, trade secrets and contractual provisions that we rely on to protect our intellectual property is inadequate, our ability to successfully commercialize our marketed products and product candidates will be harmed, and we may not be able to operate our business profitably.
Our success and ability to compete is dependent, in part, upon our ability to maintain the proprietary nature of our technologies. We rely on a combination of patent, copyright, trademark and trade secret law and nondisclosure agreements to protect our intellectual property. However, such methods may not be adequate to protect us or permit us to gain or maintain a competitive advantage. Our patent applications may not issue as patents in a form that will be advantageous to us, or at all. Our issued patents, and those that may issue in the future, may be challenged, invalidated or circumvented, which could limit our ability to stop competitors from marketing related products.
20
To protect our proprietary rights, we may in the future need to assert claims of infringement against third parties to protect our intellectual property. There can be no assurance that we will be successful on the merits in any enforcement effort. In addition, we may not have sufficient resources to litigate, enforce or defend our intellectual property rights. Litigation to enforce our intellectual property rights in patents, copyrights or trademarks is highly unpredictable, expensive and time consuming and would divert human and monetary resources away from managing our business, all of which could have a material adverse effect on our financial condition and results of operations even if we were to prevail in such litigation. In the event of an adverse judgment, a court could hold that some or all of our asserted intellectual property rights are not infringed, or that they are invalid or unenforceable, and could award attorney fees.
Despite our efforts to safeguard our unpatented and unregistered intellectual property rights, we may not be successful in doing so or the steps taken by us in this regard may not be adequate to detect or deter misappropriation of our technologies or to prevent an unauthorized third party from copying or otherwise obtaining and using our products, technologies or other information that we regard as proprietary. Additionally, third parties may be able to design around our patents. Furthermore, the laws of foreign countries may not protect our proprietary rights to the same extent as the laws of the United States. Our inability to adequately protect our intellectual property could allow our competitors and others to produce products based on our technologies, which could substantially impair our ability to compete.
We have entered into confidentiality and intellectual property assignment agreements with our employees and consultants as one of the ways we seek to protect our intellectual property and other proprietary technologies. However, these agreements may not be enforceable or may not provide meaningful protection for our trade secrets or other proprietary information in the event of unauthorized use or disclosure or other breaches of the agreements.
Our employees and consultants may unintentionally or willfully disclose our confidential information to competitors, and confidentiality agreements may not provide an adequate remedy in the event of unauthorized disclosure of confidential information. Enforcing a claim that a third party illegally obtained and is using our proprietary know-how is expensive and time-consuming, and the outcome is unpredictable. In addition, courts outside the United States are sometimes less willing to protect know-how than courts in the United States. Moreover, our competitors may independently develop equivalent knowledge, methods and know-how. Failure to obtain or maintain intellectual property protection could adversely affect our competitive business position.
We may be dependent upon one of our licenses from The Johns Hopkins University to develop and commercialize some components of the ClearTrace system.
We have entered into exclusive license agreements with The Johns Hopkins University, or Johns Hopkins, with respect to a number of technologies owned by Johns Hopkins. Under one of those agreements, which we entered into in 1998, we licensed a number of technologies relating to devices, systems and methods for performing MRI-guided interventions, particularly MRI-guided cardiac ablation procedures. Therefore, that license is important to the development of the ClearTrace system. Without that license, we may not be able to commercialize some of the components of the ClearTrace system when, and if, developed, subject to FDA clearance or approval. Johns Hopkins has the right to terminate the license under specified circumstances, including a breach by us and failure to cure such breach or in the event we file for bankruptcy. We are obligated to use commercially reasonable efforts to develop and commercialize products based on the licensed patents and patent applications. This obligation could require us to take actions related to the development of the ClearTrace system that we would otherwise not take.
21
Risks Related to Regulatory Compliance
We operate in a highly-regulated industry and any failure to comply with the extensive government regulations may subject us to fines, injunctions and other penalties that could harm our business.
Our marketed products, our product candidates and operations are subject to extensive regulation by the FDA and various other federal, state and foreign governmental authorities. Government regulations and foreign requirements specific to medical devices are wide ranging and govern, among other things:
| • | design, development and manufacturing; |
| • | testing, labeling and storage; |
| • | product safety; |
| • | marketing, sales and distribution; |
| • | premarket clearance or approval; |
| • | recordkeeping procedures; |
| • | advertising and promotions; |
| • | recalls and field corrective actions; |
| • | post-market surveillance, including reporting of deaths or serious injuries and malfunctions that, if they were to recur, could lead to death or serious injury; and |
| • | product export. |
We are subject to ongoing FDA requirements, including: required submissions of safety and other post-market information; manufacturing facility registration and device listing requirements; compliance with FDA’s medical device current Good Manufacturing Practice regulations, as codified in the Quality System Regulation, or QSR; requirements regarding field corrections and removals of our marketed products; reporting of adverse events and certain product malfunctions to the FDA; and numerous recordkeeping requirements. If we or any of our collaborators or suppliers fail to comply with applicable regulatory requirements, a regulatory agency may take action against us, including any of the following sanctions:
| • | untitled letters, warning letters, fines, injunctions, consent decrees and civil penalties; |
| • | customer notifications or orders for the repair or replacement of our marketed products or refunds; |
| • | recall, detention or seizure of our marketed products; |
| • | operating restrictions or partial suspension or total shutdown of production; |
| • | refusing or delaying requests for 510(k) clearances or PMA approvals of new products or modified products; |
| • | withdrawing 510(k) clearances or PMA approvals that have already been granted; or |
| • | refusing to grant export approval for our marketed products. |
The FDA’s and foreign regulatory agencies’ statutes, regulations or policies may change, and additional government regulation or statutes may be enacted, which could increase post-approval regulatory requirements, or delay, suspend or prevent marketing of our marketed products. We cannot predict the likelihood, nature or extent of adverse governmental regulation that might arise from future legislative or administrative action, either in the United States or abroad.
22
If we or our third-party suppliers fail to comply with the FDA’s QSR or any applicable state equivalent, our manufacturing operations could be interrupted and our potential product sales and operating results could suffer.
We and some of our third-party suppliers are required to comply with the FDA’s QSR, which covers the methods and documentation of the design, testing, production, control, quality assurance, labeling, packaging, sterilization, storage and shipping of our marketed products and product candidates. We and our suppliers will also be subject to the regulations of foreign jurisdictions regarding the manufacturing process if we market our products in these jurisdictions. The FDA enforces the QSR through periodic and unannounced inspections of manufacturing facilities. Our facilities have not been inspected by the FDA for QSR compliance. We anticipate that we and certain of our third-party suppliers will be subject to future inspections. The failure by us or one of our third-party suppliers to comply with applicable statutes and regulations administered by the FDA and other regulatory bodies, or the failure to timely and adequately respond to any adverse inspectional observations, could result in enforcement actions against us, which could impair our ability to produce our marketed products in a cost-effective and timely manner in order to meet our customers’ demands. If we fail to comply with the FDA’s QSR or any applicable state equivalent, we would be required to incur the costs and take the actions necessary to bring our operations into compliance, which may have a negative impact on our future sales and our ability to generate a profit.
Our marketed products may in the future be subject to product recalls that could harm our reputation, business operations and financial results.
The FDA and similar foreign governmental authorities have the authority to require the recall of commercialized products in the event of material deficiencies or defects in design, manufacture or labeling. In the case of the FDA, the authority to require a recall must be based on an FDA finding that there is a reasonable probability that the device would cause serious injury or death. In addition, foreign governmental bodies have the authority to require the recall of our marketed products in the event of material deficiencies or defects in design or manufacture. Manufacturers may, under their own initiative, recall a product if any material deficiency in a device is found. A government-mandated or voluntary recall by us could occur as a result of component failures, manufacturing errors, design or labeling defects or other deficiencies and issues. Recalls of any of our marketed products would divert managerial and financial resources and have an adverse effect on our financial condition and results of operations. We may initiate certain voluntary recalls involving our marketed products in the future. Companies are required to maintain certain records of recalls, even if they are not reportable to the FDA. If we determine that certain of those recalls do not require notification to the FDA, the FDA may disagree with our determinations and require us to report those actions as recalls. A future recall announcement could harm our reputation with customers and negatively affect our sales. In addition, the FDA could take enforcement actions against us, which could impair our ability to produce our marketed products in a cost-effective and timely manner in order to meet our customers’ demands. Regulatory investigations or product recalls could also result in our incurring substantial costs, losing revenues and implementing a change in the design, manufacturing process or the indications for which our products may be used, each of which would harm our business.
If our marketed products cause or contribute to a death or a serious injury, or malfunction in certain ways, we will be subject to medical device reporting regulations, which can result in voluntary corrective actions or agency enforcement actions.
Under the FDA’s medical device reporting regulations, we are required to report to the FDA any incident in which our marketed products may have caused or contributed to a death or serious injury or in which our marketed products malfunctioned and, if the malfunction were to recur, would likely cause or contribute to death or serious injury. In the future, we may experience events that may require reporting to the FDA pursuant to the medical device reporting regulations. In addition, all manufacturers placing medical devices in European Union markets are legally bound to report any serious or potentially serious incidents involving devices they produce or sell to the relevant authority in whose jurisdiction the incident occurred. Any adverse event involving our
23
marketed products could result in future voluntary corrective actions, such as recalls or customer notifications, or agency action, such as inspection, mandatory recall or other enforcement action. Any corrective action, whether voluntary or involuntary, as well as defending ourselves in a lawsuit, will require the dedication of our time and capital, distract management from operating our business, and may harm our reputation and financial results. In addition, failure to report such adverse events to appropriate government authorities on a timely basis, or at all, could result in an enforcement action against us.
We may incur significant liability if it is determined that we are promoting off-label uses of our products in violation of federal and state regulations in the United States or elsewhere.
We obtained 510(k) clearance of our ClearPoint system from the FDA for a general neurological intervention claim. This general neurological intervention indication is the same indication for use that applies to other devices that have traditionally been used in the performance of stereotactic neurological procedures, under which a physician merges pre-operative images and data with specialized surgical instruments to help guide the procedure. Our business and future growth, however, may depend substantially on the use or enhancement of our ClearPoint system in deep brain stimulation lead placement procedures. In the future, we may seek regulatory clearance or approval, as the case may be, for use of our ClearPoint system for a variety of specific neurological indications, including deep brain stimulation lead placement, to allow us to market and promote our ClearPoint system for those specific uses. Unless and until we receive regulatory clearance or approval for use of our ClearPoint system in these specific procedures, uses in procedures other than general neurological intervention procedures, such as biopsies and catheter and electrode insertion, may be considered off-label uses of our ClearPoint system.
Under the federal Food, Drug, and Cosmetic Act and other similar laws, we are prohibited from labeling or promoting our ClearPoint system, or training physicians, for such off-label uses. The FDA defines labeling to include not only the physical label attached to the product, but also items accompanying the product. This definition also includes items as diverse as materials that appear on a company’s website. As a result, we are not permitted to promote uses of our marketed products that are not cleared or approved, whether on our website, in product brochures or in customer communications. This prohibition means that the FDA could deem it unlawful for us to make claims about the use of our ClearPoint system specifically for deep brain stimulation lead placement procedures or proactively discuss or provide information or training on the use of our ClearPoint system specifically for deep brain stimulation lead placement procedures. However, although manufacturers are not permitted to promote for off-label uses, in their practice of medicine, physicians may lawfully choose to use medical devices for off-label uses. Therefore, a physician could use our ClearPoint system for uses not covered by the cleared labeling. This would constitute an off-label use. We expect that physicians will use our ClearPoint system for a variety of specific neurological procedures, including deep brain stimulation lead placement.
The FDA and other regulatory agencies actively enforce regulations prohibiting promotion of off-label uses and the promotion of products for which marketing clearance or approval has not been obtained. If the FDA determines that our promotional materials or training constitutes promotion of an off-label use, it could request that we modify our training or promotional materials or subject us to regulatory or enforcement actions, including the issuance of an untitled letter, warning letter, injunction, seizure, civil fine and criminal penalties. It is also possible that other federal, state or foreign enforcement authorities might take action if they consider our promotional or training materials to constitute promotion of an unapproved use, which could result in significant fines or penalties under other statutory authorities, such as laws prohibiting false claims for reimbursement. In that event, our reputation could be damaged and market adoption of our marketed products would be impaired. Due to these legal constraints, our sales and marketing efforts will focus only on the general technical attributes and benefits of our ClearPoint system and the FDA cleared indications for use. In addition, the off-label use of our marketed products may increase the risk of injury to patients, and, in turn, the risk of product liability claims. Product liability claims are expensive to defend and could divert our management’s attention and result in substantial damage awards against us.
24
We may be subject, directly or indirectly, to federal and state healthcare fraud and abuse laws and regulations and could face substantial penalties if we are unable to fully comply with such laws.
Although we do not provide healthcare services or receive payments directly from Medicare, Medicaid or other third-party payors for our marketed products or the procedures in which our marketed products may be used, many state and federal healthcare laws and regulations governing financial relationships between medical device companies and healthcare providers apply to our business and we could be subject to enforcement by both the federal government, private whistleblowers and the states in which we conduct our business. The healthcare laws and regulations that may affect our ability to operate include:
| • | The federal healthcare programs’ Anti-Kickback Statute, which prohibits, among other things, individuals or entities from knowingly and willfully soliciting, receiving, offering or providing any kickback, bribe or other remuneration, directly or indirectly, in exchange for or to induce the purchase, lease or order, or arranging for or recommending of, any item or service for which payment may be made under a federal healthcare program such as the Medicare and Medicaid programs. |
| • | Federal false claims laws, which prohibit, among other things, individuals or entities from knowingly presenting, or causing to be presented, claims for payment to Medicare, Medicaid or other federally- funded healthcare programs that are false or fraudulent, or are for items or services not provided as claimed, and which may apply to entities like us to the extent that our interactions with customers may affect their billing or coding practices. Changes to the Federal false claims law enacted as part of the Health Care Reform Law will likely increase the number of whistleblower cases brought against providers and suppliers of health care items and services. |
| • | The federal Health Insurance Portability and Accountability Act of 1996, or HIPAA, in addition to the privacy and security rules normally associated with it, which are discussed below, established new federal crimes for knowingly and willfully executing a scheme to defraud any healthcare benefit program or making false statements in connection with the delivery of or payment for healthcare benefits, items or services. |
| • | State and foreign law equivalents and analogues of each of the above federal laws, such as anti-kickback and false claims laws and the Foreign Corrupt Practices Act, which may apply to items or services reimbursed by any third-party payor, including commercial insurers, or when physicians are employees of a foreign government entity. |
| • | The Health Care Reform Law, which imposes certain reporting obligations on manufacturers of drugs, devices and biologics. On March 31, 2013, and on the 90th day of each calendar year thereafter, these manufacturers must report all payments or other transfers of value to or on behalf of a physician or teaching hospital by such manufacturers as well as any ownership or investment interest held by physicians in such manufacturers. The Health Care Reform Law also grants the Office of Inspector General additional authority to obtain information from any individual or entity to validate claims for payment or to evaluate the economy, efficiency or effectiveness of the Medicare and Medicaid programs, expands the permissible exclusion authority to include any false statements or misrepresentations of material facts, enhances the civil monetary penalties for false statements or misrepresentation of material facts, and enhances the Federal Sentencing Guidelines for those convicted of Federal healthcare offenses. |
Recently, the medical device industry has been under heightened scrutiny as the subject of government investigations and government enforcement or private whistleblower actions under the Anti-Kickback Statute and the False Claims Act involving manufacturers who allegedly offered unlawful inducements to potential or existing customers in an attempt to procure their business, including specifically arrangements with physician consultants. For example, in 2007, four of the five major orthopedic implant manufacturers were required to pay a total of $311 million and operate for 18 months under federal court supervision in settlement of kickback allegations concerning their physician consulting contracts.
25
We have a number of agreements with physicians that may be scrutinized or may be subject to reporting requirements in the future, including consulting contracts for product development in which we compensate physicians for various services, including:
| • | keeping us informed of new developments in their respective fields of practice; |
| • | advising us on our research and development projects related to their respective fields; |
| • | advising us on improvements to methods, processes and devices related to their respective fields (such as advice on the development of prototype devices); |
| • | assisting us with the technical evaluation of our methods, processes and devices related to their respective fields; and |
| • | advising us with respect to the commercialization of products in their respective fields. |
We may enter into similar consulting agreements with physicians in the future. Likewise, we may enter into consulting agreements with physicians to provide training and other similar services on the proper use of our marketed products.
The Health Care Reform Law mandates increased transparency of arrangements between physicians and medical device companies, which we expect will increase our overall cost of compliance. We believe that this increased transparency will also result in a heightened level of government scrutiny of the relationships between physicians and medical device companies. While we believe that all of our arrangements with physicians comply with applicable law, the increased level of scrutiny, coupled with the expanded enforcement tools available to the government under the Health Care Reform Law, may increase the likelihood of a governmental investigation. If we become subject to such an investigation, our business and operations would be adversely affected even if we ultimately prevail because the cost of defending such investigation would be substantial. Moreover, companies subject to governmental investigations could lose both overall market value and market share during the course of the investigation.
In addition, we may provide customers with information on products that could be deemed to influence their coding or billing practices, and may have sales, marketing or other arrangements with hospitals and other providers that could also be the subject of scrutiny under these laws. If our operations are found to be in violation of any of the laws described above or any other governmental regulations that apply to us, we may be subject to penalties, including civil and criminal penalties, damages, fines, exclusion from the Medicare and Medicaid programs and the curtailment or restructuring of our operations. Any penalties, damages, fines, exclusions, curtailment or restructuring of our operations could adversely affect our ability to operate our business and our financial results. The risk of our being found in violation of these laws is increased by the fact that many of these laws are broad and their provisions are open to a variety of interpretations. Any action against us for violation of these laws, even if we successfully defend against it, could cause us to incur significant legal expenses and divert our management’s attention from the operation of our business. If the surgeons or other providers or entities with whom we do business are found to be non-compliant with applicable laws, they may be subject to sanctions, which could also have a negative impact on our business.
We may be subject to privacy and data protection laws governing the transmission, security and privacy of health information which may impose restrictions on technologies and subject us to penalties if we are unable to fully comply with such laws.
Numerous federal, state and international laws and regulations govern the collection, use, disclosure, storage and transmission of patient-identifiable health information. These laws include:
| • | HIPAA and its implementing regulations, the HIPAA Privacy and Security Rules, apply to covered entities, which include most healthcare facilities that purchase and use our products. The HIPAA Privacy and Security Rules set forth minimum standards for safeguarding individually identifiable |
26
| health information, impose certain requirements relating to the privacy, security and transmission of individually identifiable health information and provide certain rights to individuals with respect to that information. HIPAA also requires covered entities to contractually bind third parties, known as business associates, in the event that they perform an activity or service for or on behalf of the covered entity that involves access to patient identifiable health information, including imposing liability on business associates of HIPAA “covered entities”. |
| • | The federal Health Information Technology for Economic and Clinical Health Act, or HITECH, which was enacted in February 2009, strengthens and expands the HIPAA Privacy and Security Rules and its restrictions on use and disclosure of patient identifiable health information. |
| • | Other federal and state laws restricting the use and protecting the privacy and security of patient information may apply, many of which are not preempted by HIPAA. |
| • | Federal and state consumer protection laws are being applied increasingly by the United States Federal Trade Commission, or FTC, and state attorneys’ general to regulate the collection, use, storage and disclosure of personal or patient information, through websites or otherwise, and to regulate the presentation of website content. |
| • | Other countries also have, or are developing, laws governing the collection, use and transmission of personal or patient information. |
| • | Federal and state laws regulating the conduct of research with human subjects. |
We are required to comply with federal and state laws governing the transmission, security and privacy of patient identifiable health information that we may obtain or have access to in connection with manufacture and sale of our marketed products. We do not believe that we are a HIPAA covered entity because we do not submit electronic claims to third-party payors, but there may be limited circumstances in which we may operate as a business associate to covered entities if we receive patient identifiable data through activities such as training providers on the use of our marketed products or investigating product performance or if our marketed products store patient identifiable health information on behalf of a healthcare provider. We may be required to make costly system modifications to comply with the HIPAA privacy and security requirements that will be imposed on us contractually through business associate agreements by covered entities and directly under HITECH provisions that became effective in February 2010. Due to the recent enactment of HITECH, we are not able to predict what the extent of the impact on our business may be. Our failure to comply may result in criminal and civil liability because the potential for enforcement action against business associates is now greater. Enforcement actions can be costly and interrupt regular operations which may adversely affect our business.
In addition, numerous other federal and state laws protect the confidentiality of patient information as well as employee personal information, including state medical privacy laws, state social security number protection laws and federal and state consumer protection laws. These various laws in many cases are not preempted by the HIPAA rules and may be subject to varying interpretations by the courts and government agencies, creating complex compliance issues for us and our customers and potentially exposing us to additional expense, adverse publicity and liability.
In connection with any clinical trials we conduct, we will be subject to state and federal privacy and human subject protection regulations. The HIPAA requirement and other human subjects research laws could create liability for us or increase our cost of doing business because we must depend on our research collaborators to comply with the applicable laws. We may adopt policies and procedures that facilitate our collaborators’ compliance, but we cannot ensure that non-employee collaborators or investigators will comply with applicable laws. As a result, unauthorized uses and disclosures of research subject information in violation of the law may occur. These violations may lead to sanctions that will adversely affect our business.
27
Risks Related to Facilities, Employees and Growth
We are dependent on our senior management team, engineering team, sales and marketing team and key research and physician advisors, and the loss of any of them could harm our business.
We are highly dependent on members of our senior management, in particular Kimble L. Jenkins, our President, Chief Executive Officer and Chairman of the Board of Directors, and Peter G. Piferi, our Chief Operating Officer. The loss of members of our senior management team, engineering team, sales and marketing team and key research and physician advisors, or our inability to attract or retain other qualified personnel or advisors, could have a material adverse effect on our business, financial condition and results of operations. We do not maintain key employee life insurance on any of our personnel other than for Mr. Jenkins and Mr. Piferi. Although we have obtained key employee insurance covering Mr. Jenkins and Mr. Piferi in the amount of $2,000,000, this would not fully compensate us for the loss of Mr. Jenkins’ or Mr. Piferi’s services.
We adopted our Key Personnel Incentive Plan, which is described in more detail on page 112, to provide Dr. Paul Bottomley, who is a key research advisor, and Mr. Parag Karmarkar, who is a key member of our engineering team, the opportunity to receive incentive bonus payments based on future performance of services to us or upon a sale of our company. However, if Dr. Bottomley or Mr. Karmarkar dies, becomes disabled or is involuntarily terminated by us without cause, we nevertheless would be obligated to make the incentive bonus payments otherwise provided under the plan. The obligation to make these payments could have a material adverse effect on our financial position. We may obtain life insurance on Dr. Bottomley and Mr. Karmarkar to reduce our financial exposure in the event of a participant’s death. We also adopted the Cardiac EP Business Participation Plan, which is described in more detail on page 112, to provide Dr. Nassir Marrouche, who is a key product development advisor, with financial rewards in the event that we sell our business operations relating to catheter-based MRI-guided cardiac ablation to treat cardiac arrhythmias, which we refer to as our cardiac EP business operations. If we sell our cardiac EP business operations or our entire company, we will be required to make a payment to Dr. Marrouche which is calculated as a percentage of the purchase price paid for, or allocated to, our cardiac EP business operations.
We need to hire and retain additional qualified personnel to grow and manage our business. If we are unable to attract and retain qualified personnel, our business and growth could be seriously harmed.
Our performance depends on the talents and efforts of our employees. Our future success will depend on our ability to attract, retain and motivate highly skilled personnel in all areas of our organization. We plan to continue to grow our business and will need to hire additional personnel to support this growth. We believe that there are only a limited number of individuals with the requisite skills to serve in many of our key positions, and we compete for key personnel with other medical device companies, as well as universities and research institutions. It is often difficult to hire and retain these persons, and we may be unable to replace key persons if they leave or fill new positions requiring key persons with appropriate experience. If we experience difficulties locating and hiring suitable personnel in the future, our growth may be hindered. Qualified individuals are in high demand, particularly in the medical device industry, and we may incur significant costs to attract and retain them. Employees that hold shares of our common stock or options to purchase our common stock may be more likely to leave us following our initial public offering as a result of the establishment of a public market for our common stock. If we are unable to attract and retain the personnel we need to succeed, our business and growth could be harmed.
28
If we do not effectively manage our growth, we may be unable to successfully develop, market and sell our products.
Our future revenue and operating results will depend on our ability to manage the anticipated growth of our business. In order to achieve our business objectives, we must continue to grow. However, continued growth presents numerous challenges, including:
| • | implementing appropriate operational and financial systems and controls; |
| • | expanding our assembly capacity and increasing production through third parties; |
| • | expanding our sales and marketing infrastructure and capabilities; |
| • | improving our information systems; |
| • | identifying, attracting and retaining qualified personnel in our areas of activity; and |
| • | hiring, training, managing and supervising our personnel. |
We cannot be certain that our systems, controls, infrastructure and personnel will be adequate to support our future operations. Any failure to effectively manage our growth could impede our ability to successfully develop, market and sell our products and our business will be harmed.
Our operations are vulnerable to interruption or loss due to natural disasters, power loss and other events beyond our control, which would adversely affect our business.
We will conduct a significant portion of our activities, including component processing, final assembly, packaging and distribution activities for our ClearPoint system, at a facility located in Irvine, California, which is a seismically active area that has experienced major earthquakes in the past, as well as other natural disasters, including wildfires. We have taken precautions to safeguard our facility, including obtaining business interruption insurance. However, any future natural disaster, such as an earthquake or a wildfire, could significantly disrupt our operations, and delay or prevent product assembly and shipment during the time required to repair, rebuild or replace our facility, which could be lengthy and result in significant expenses. Furthermore, the insurance coverage we maintain may not be adequate to cover our losses in any particular case or continue to be available at commercially reasonable rates and terms. In addition, our facility may be subject to shortages of electrical power, natural gas, water and other energy supplies. Any future shortage or conservation measure could disrupt our operations and cause expense, thus adversely affecting our business and financial results.
Risks Related to this Offering
An active trading market for our common stock may not develop.
Prior to this offering, there has been no public market for our common stock. We have been approved for listing on the Nasdaq Capital Market; however, an active trading market for our shares may never develop or be sustained following this offering. Accordingly, you may not be able to sell your shares quickly or at the market price if trading in our stock is not active.
Market volatility may cause our stock price and the value of your investment to decline.
The initial public offering price for our common stock was determined through negotiations between the underwriters and us. The initial public offering price may vary from the market price of our common stock after the closing of this offering. Investors may not be able to sell their common stock at or above the initial public offering price.
29
We expect that the price of our common stock will fluctuate substantially, as the market price for our common stock after this offering will be affected by a number of factors, including:
| • | the receipt, denial or timing of regulatory clearances or approvals of our product candidates or competing products; |
| • | changes in policies affecting third-party coverage and reimbursement in the United States and other countries; |
| • | ability of our marketed products or our product candidates, if they receive regulatory clearance or approval, to achieve market success; |
| • | the performance of third-party contract manufacturers and component suppliers; |
| • | our ability to enhance our sales and marketing capabilities; |
| • | our ability to manufacture our marketed products to commercial standards; |
| • | the success of any collaborations we have or may undertake with other companies; |
| • | our ability to develop, introduce and market new or enhanced versions of our marketed products on a timely basis; |
| • | actual or anticipated variations in our results of operations or those of our competitors; |
| • | announcements of new products, technological innovations or product advancements by us or our competitors; |
| • | sales of common stock or other securities by us or our stockholders in the future; |
| • | additions or departures of key management personnel; |
| • | disputes or other developments relating to proprietary rights, including patents, litigation matters and our ability to obtain patent protection for our technologies; |
| • | trading volume of our common stock; |
| • | changes in earnings estimates or recommendations by securities analysts, failure to obtain analyst coverage of our common stock or our failure to achieve analyst earnings estimates; |
| • | developments in our industry; and |
| • | general market conditions and other factors unrelated to our operating performance or the operating performance of our competitors. |
In addition, the stock prices of many companies in the medical device industry have experienced wide fluctuations that have often been unrelated to the operating performance of these companies. We expect our stock price to be similarly volatile. These broad market fluctuations may continue and could harm our stock price. Following periods of volatility in the market price of a company’s securities, stockholders have often instituted class action securities litigation against those companies. Class action securities litigation, if instituted against us, could result in substantial costs and a diversion of our management resources, which could significantly harm our business.
Securities analysts may not initiate coverage for our common stock or may issue negative reports, and this may have a negative impact on the market price of our common stock.
Securities analysts may elect not to provide research coverage of our common stock after the completion of this offering. The lack of research coverage may adversely affect the market price of our common stock. The trading market for our common stock may be affected in part by the research and reports that industry or financial analysts publish about us or our business. It may be difficult for companies such as ours, with smaller market capitalizations, to attract securities analysts that will cover our common stock. If one or more of the
30
analysts who elects to cover us downgrades our stock, our stock price would likely decline rapidly. If one or more of these analysts ceases coverage of our company, we could lose visibility in the market, which in turn could cause our stock price to decline. This could have a negative effect on the market price of our stock.
Our directors, executive officers and principal stockholders and their respective affiliates will continue to have substantial control over us after this offering and could delay or prevent a change in corporate control.
After this offering, our directors, executive officers and holders of more than 5% of our common stock, together with their affiliates, will beneficially own, in the aggregate, approximately 23.4% of our outstanding common stock. As a result, these stockholders, acting together, will continue to have substantial control over the outcome of matters submitted to our stockholders for approval, including the election of directors and any merger, consolidation or sale of all or substantially all of our assets. In addition, these stockholders, acting together, will continue to have significant influence over the management and affairs of our company. Accordingly, this concentration of ownership may have the effect of:
| • | delaying, deferring or preventing a change in corporate control; |
| • | impeding a merger, consolidation, takeover or other business combination involving us; or |
| • | discouraging a potential acquirer from making a tender offer or otherwise attempting to obtain control of us. |
We have not paid dividends in the past and do not expect to pay dividends in the future.
We have never declared or paid cash dividends on our capital stock. We currently intend to retain all future earnings for the operation and expansion of our business and, therefore, do not anticipate declaring or paying cash dividends in the foreseeable future. The payment of dividends will be at the discretion of our Board of Directors and will depend on our results of operations, capital requirements, financial condition, prospects, contractual arrangements, any limitations on payments of dividends present in any of our future debt agreements and other factors our Board of Directors may deem relevant. If we do not pay dividends, a return on your investment will only occur if our stock price appreciates.
Anti-takeover provisions in our certificate of incorporation, bylaws and Delaware law could prevent or delay a change in control of our company.
Provisions in our certificate of incorporation and bylaws, which will be effective upon the closing of this offering, as well as provisions of Delaware law, may discourage, delay or prevent a merger, acquisition or change of control. These provisions could also discourage proxy contests and make it more difficult for stockholders to elect directors and take other corporate actions. These provisions:
| • | provide for a staggered Board of Directors; |
| • | permit our Board of Directors to issue shares of preferred stock, with any rights, preferences and privileges as they may designate, including the right to approve an acquisition or other change in our control; |
| • | provide that the authorized number of directors may be changed only by resolution of the Board of Directors; |
| • | provide that all vacancies, including newly created directorships, may, except as otherwise required by law, be filled by the affirmative vote of a majority of directors then in office, even if less than a quorum; |
| • | require that any action to be taken by our stockholders must be effected at a duly called annual or special meeting of stockholders and not be taken by written consent; |
31
| • | provide that stockholders seeking to present proposals before a meeting of stockholders or to nominate candidates for election as directors at a meeting of stockholders must provide notice in writing in a timely manner, and also specify requirements as to the form and content of a stockholder’s notice; |
| • | do not provide for cumulative voting rights (therefore allowing the holders of a majority of the shares of common stock entitled to vote in any election of directors to elect all of the directors standing for election, if they should so choose); |
| • | provide that special meetings of our stockholders may be called only by the chairman of the Board of Directors, our Chief Executive Officer or by the Board of Directors pursuant to a resolution adopted by a majority of the total number of authorized directors; and |
| • | provide that stockholders will be permitted to amend our amended and restated bylaws only upon receiving at least 66 2/3% of the votes entitled to be cast by holders of all outstanding shares then entitled to vote generally in the election of directors, voting together as a single class. |
In addition, we are subject to Section 203 of the Delaware General Corporation Law, which generally prohibits a Delaware corporation from engaging in any broad range of business combinations with any stockholder who owns, or at any time in the last three years owned, 15% or more of our outstanding voting stock for a period of three years following the date on which the stockholder became an interested stockholder. This provision could have the effect of delaying or preventing a change of control, whether or not it is desired by or beneficial to our stockholders.
Sales of a substantial number of shares of our common stock in the public market after this offering, or the perception that they may occur, may depress the market price of our common stock.
Sales of substantial amounts of our common stock in the public market following this offering, or the perception that substantial sales may be made, could cause the market price of our common stock to decline. The lock-up agreements to be delivered by our executive officers, directors and certain of our stockholders provide that the underwriters, acting jointly and in their discretion, may release those parties, at any time, or from time to time, and without notice, from their obligation not to dispose of shares of common stock for a period of 180 days after the date of this prospectus, which period may be extended in certain limited circumstances. The underwriters do not have any pre-established conditions to waiving the terms of the lock-up agreements, and any decision by them to waive those conditions would depend on a number of factors, which may include market conditions, the performance of the common stock in the market and our financial condition at that time.
Based on the number of shares of common stock outstanding as of April 30, 2010, upon completion of this offering, 10,129,405 shares of our common stock will be outstanding. All of the shares sold in this offering will be freely transferable unless held by an affiliate of ours. Of the remaining shares, during the 90 days following the date of this prospectus, 1,965,521 shares held by non-affiliates, or approximately 19.4% of our common stock outstanding after this offering, will be freely transferable subject to compliance with Rule 144. Beginning on the 91st day following the date of this prospectus, 2,474,396 shares of our common stock, or approximately 24.4% of our common stock outstanding after this offering, will be freely transferable subject to compliance with Rule 144 under the Securities Act. The lockup agreements between the underwriters and our directors, executive officers and certain of our stockholders will expire 180 days after the date of this prospectus, at which time all of our shares of our common stock will be freely transferable subject to compliance with the provisions of Rule 144 under the Securities Act. See “Shares Eligible for Future Sale—Lock-up Agreements.” Our affiliates must comply with the volume, manner of sale, holding period and other limitations of Rule 144. As restrictions on resale end, the market price could drop significantly if the holders of these restricted shares sell them or are perceived by the market as intending to sell them. Any substantial sale of common stock pursuant to any resale registration statements or Rule 144 may have an adverse effect on the market price of our common stock by creating an excessive supply.
We intend to file a registration statement on Form S-8 to register the 1,850,625 shares subject to outstanding options or reserved for issuance under our stock option plans. The registration statement will become effective
32
when filed, and, subject to applicable lock-up agreements, if any, these shares may be resold without restriction in the public marketplace. For a more detailed description, please see the section of this prospectus entitled “Shares Eligible for Future Sale.”
New investors in our common stock will experience immediate and substantial dilution after this offering.
If you purchase shares of our common stock in this offering, you will experience immediate dilution of $8.82 per share based on the mid-point of the range on the cover of this prospectus because the price that you pay will be substantially greater than the adjusted pro forma net tangible book value per share of common stock that you acquire. This dilution is due in large part to the fact that many of our earlier investors paid substantially less than the price of the shares being sold in this offering when they purchased their shares of our capital stock. If outstanding options and warrants to purchase our common stock are exercised, you will experience additional dilution. See the section entitled “Dilution” in this prospectus for a more detailed description of this dilution.
We will incur significant increased costs as a result of operating as a public company, and our management will be required to divert attention from product development and commercialization and to devote substantial resources and time to new compliance initiatives.
As a public company, we will incur significant legal, accounting and other expenses that we did not incur as a private company. We are working with our independent legal and accounting advisors to identify those areas in which changes should be made to our financial and management control systems to manage our growth and our obligations as a public company. These areas include corporate control, disclosure controls and procedures and financial reporting and accounting systems, including requirements under the Sarbanes-Oxley Act of 2002, as amended, or the Sarbanes-Oxley Act. We will incur costs associated with our public company reporting requirements and corporate governance requirements, including requirements under the Sarbanes-Oxley Act, as well as rules implemented by the SEC and the securities exchange on which our stock trades. We will need to devote a substantial amount of time to these compliance initiatives. Moreover, these rules and regulations will increase our legal and financial compliance costs and will make some activities more time-consuming and costly. For example, we expect these rules and regulations to make it more difficult and more expensive for us to obtain director and officer liability insurance, and we may be required to accept reduced policy limits and coverage or incur substantially higher costs to obtain the same or similar coverage. As a result, it may be more difficult for us to attract and retain qualified people to serve on our Board of Directors, our board committees or as executive officers.
In addition, the Sarbanes-Oxley Act requires, among other things, that we maintain effective internal control over financial reporting and disclosure controls and procedures. In particular, for the fiscal year ending December 31, 2010, we must perform system and process evaluation and testing of our internal control over financial reporting to allow management and our independent registered public accounting firm to report on the effectiveness of our internal control over financial reporting, as required by Section 404 of the Sarbanes-Oxley Act. Our compliance with Section 404 will require that we incur substantial expense and expend significant management time on compliance-related issues.
We may not use the net proceeds from this offering effectively.
We intend to use the net proceeds from this offering in the manner described in “Use of Proceeds” elsewhere in this prospectus. Our use of the net proceeds of the offering in this manner will not necessarily improve our operating results or enhance the value of our common stock. The failure by our management to apply these funds effectively could result in financial losses, cause the price of our common stock to decline or delay product development.
33
SPECIAL NOTE REGARDING FORWARD-LOOKING STATEMENTS
This prospectus contains forward-looking statements. The forward-looking statements are contained principally in the sections entitled “Prospectus Summary,” “Risk Factors,” “Use of Proceeds,” “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and “Business.” These statements involve known and unknown risks, uncertainties and other factors that may cause our actual results, performance or achievements to be materially different from any future results, performances or achievements, expressed or implied by the forward-looking statements. Forward-looking statements include, but are not limited to, statements about:
| • | the ability to market, commercialize and achieve market acceptance for our marketed products, current product candidates or any future product candidates; |
| • | the ability to obtain regulatory clearance or approval for our current product candidates or any future product candidates; |
| • | the anticipated progress of our research, development and clinical trials; |
| • | the ability to generate additional product candidates; |
| • | the ability to protect our intellectual property and operate our business without infringing upon the intellectual property rights of others; and |
| • | the estimates regarding the sufficiency of our cash resources. |
In some cases, you can identify forward-looking statements by terms such as “anticipates,” “believes,” “could,” “estimates,” “expects,” “intends,” “may,” “plans,” “potential,” “predicts,” “projects,” “should,” “will,” “would,” and similar expressions intended to identify forward-looking statements, although not all forward-looking statements contain these words. These statements involve known and unknown risks, uncertainties and other factors that may cause our actual results, levels of activity, performance or achievements to be materially different from the information expressed or implied by these forward-looking statements. Although we believe that we have a reasonable basis for each forward-looking statement contained in this prospectus, we caution you that these statements are based on a combination of facts and factors currently known by us and our projections of the future, about which we cannot be certain.
You should refer to the section of this prospectus entitled “Risk Factors” for a discussion of important factors that may cause our actual results to differ materially from those expressed or implied by our forward-looking statements. As a result of these factors, we cannot assure you that the forward-looking statements in this prospectus will prove to be accurate. Furthermore, if our forward-looking statements prove to be inaccurate, the inaccuracy may be material. In light of the significant uncertainties in these forward-looking statements, you should not regard these statements as a representation or warranty by us or any other person that we will achieve our objectives and plans in any specified time frame, or at all. We do not undertake to update any of the forward-looking statements after the date of this prospectus, except to the extent required by applicable securities laws.
34
We estimate that our net proceeds from the sale of 2,500,000 shares of common stock in this offering will be approximately $21,300,000 and an additional $3,500,000 if the underwriters exercise their over-allotment option in full, based upon an assumed initial public offering price of $10.00 per share, which is the mid-point of the range listed on the cover of this prospectus, and after deducting estimated underwriting discounts and commissions and estimated offering expenses payable by us. The principal purposes of this offering are to obtain additional capital and to create a public market for our common stock.
A $1.00 increase (decrease) in the assumed initial public offering price of $10.00 per share would increase (decrease) the net proceeds to us from this offering by approximately $2,300,000, assuming the number of shares offered by us, as set forth on the cover of this prospectus, remains the same and after deducting estimated underwriting discounts and commissions and estimated offering expenses payable by us.
We expect to use between approximately $8,000,000 and $10,000,000 of the net proceeds from this offering to fund our research and development activities, including payments of approximately $2,200,000 to Siemens in connection with the development of the ClearTrace system software, which is only a portion of the estimated remaining development costs of the ClearTrace system, between approximately $6,000,000 and $8,000,000 to fund our sales and marketing activities and the remainder for working capital and general corporate purposes. In addition, we may use a portion of the net proceeds from this offering to acquire equipment, products or technologies, although we currently have no commitments or agreements relating to any of these types of transactions. Excluding cash generated from sales of our marketed products, we believe that the net proceeds from this offering, our existing cash resources and interest on these funds will be sufficient to meet our projected operating requirements through the end of 2011.
Pending these uses, we plan to invest the net proceeds in a variety of capital preservation instruments, including short-term, interest bearing obligations, investment grade instruments, certificates of deposit or direct or guaranteed obligations of the United States. The goal with respect to the investment of these net proceeds is capital preservation and liquidity so that such funds are readily available to fund our research and development operations.
35
We have never declared or paid any cash dividends on our capital stock. We currently intend to retain any future earnings to fund the development and expansion of our business, and therefore we do not anticipate paying cash dividends on our common stock in the foreseeable future. Any future determination to pay dividends will be at the discretion of our Board of Directors.
36
The following table sets forth our capitalization as of March 31, 2010:
| • | on an actual basis; |
| • | on a pro forma basis to reflect a 1-for-4 reverse stock split and the conversion into common stock of all outstanding shares of our preferred stock and the bridge notes; and |
| • | on a pro forma as adjusted basis to reflect the pro forma adjustments reflected above and the sale in this offering of 2,500,000 shares of common stock at an assumed initial offering price of $10.00 per share, which is the mid-point of the range listed on the cover of this prospectus, after deducting the estimated underwriting discounts and commissions and estimated offering expenses payable by us. |
The information in the following table is based on shares outstanding as of March 31, 2010 and excludes:
| • | 600,625 shares of common stock issuable upon exercise of options issued under our stock option plans, at a weighted average exercise price of $3.61 per share; |
| • | 66,652 shares of common stock issuable upon exercise of options not issued under our stock option plans, at an exercise price of $9.64 per share; |
| • | 410,542 shares of common stock issuable upon exercise of warrants, at a weighted average exercise price of $3.48 per share; |
| • | 455,208 shares of common stock issuable upon the conversion of $3,641,667 in principal amount of, and interest on, convertible promissory notes, at a conversion price of $8.00 per share; |
| • | 125,000 shares of our common stock that may be issued to the underwriters upon exercise of warrants, at an exercise price of $12.50 per share, assuming an initial public offering price of $10.00 per share, which is the mid-point of the range listed on the cover of this prospectus; |
| • | 25,444 shares of common stock that may be issued pursuant to the placement agent warrant, at an exercise price of $8.00 per share, assuming an initial public offering price of $10.00 per share, which is the mid-point of the range listed on the cover of this prospectus; |
| • | 12,500 shares of common stock issuable upon the exercise of a warrant to be issued in connection with this offering with an exercise price equal to the initial public offering price; |
| • | 541,000 shares of common stock issuable upon the exercise of options to be issued in connection with this offering under our 2010 Incentive Compensation Plan each with an exercise price equal to the initial public offering price; |
| • | 30,000 shares of common stock to be issued in connection with this offering under our 2010 Incentive Compensation Plan assuming an initial public offering price of $10.00 per share, which is the mid-point of the range listed on the cover of this prospectus; and |
| • | 679,000 shares of common stock reserved for future issuance under our 2010 Incentive Compensation Plan. |
37
You should read the information below in conjunction with the financial statements and the related notes thereto and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” included elsewhere in this prospectus.
| As of March 31, 2010 | ||||||||||||
| Actual | Pro Forma | Pro Forma As Adjusted |
||||||||||
| Convertible notes payable, net of discounts |
$ | 5,693,556 | $ | 2,436,730 | $ | 2,436,730 | ||||||
| Series A convertible preferred stock, $0.01 par value: 8,000,000 shares authorized and 7,965,000 shares issued and outstanding, actual; and no shares authorized, issued or outstanding, pro forma and pro forma as adjusted |
7,965,000 | — | — | |||||||||
| Common stock, $0.01 par value: 70,000,000 shares authorized; 5,455,110 shares issued and 5,129,280 shares outstanding, actual; 7,955,235 shares issued and 7,629,405 shares outstanding, pro forma; 10,455,235 shares issued and 10,129,405 shares outstanding, pro forma as adjusted |
54,551 | 79,552 | 104,552 | |||||||||
| Additional paid-in capital |
26,803,455 | 38,814,454 | 60,114,379 | |||||||||
| Treasury stock |
(1,679,234 | ) | (1,679,234 | ) | (1,679,234 | ) | ||||||
| Accumulated deficit |
(44,538,676 | ) | (45,352,850 | ) | (45,352,850 | ) | ||||||
| Total stockholders’ equity (deficit) |
(11,394,904 | ) | (8,138,078 | ) | 13,186,847 | |||||||
| Total capitalization |
$ | (5,701,348 | ) | $ | (5,701,348 | ) | $ | 15,623,577 | ||||
38
The historical net tangible book value of our common stock as of March 31, 2010 was approximately $(12,597,000), or $(2.46) per share, based on the number of shares of common stock outstanding as of March 31, 2010. Historical net tangible book value per share is determined by dividing our total tangible assets less total liabilities by the actual number of outstanding shares of our common stock. The pro forma net tangible book value of our common stock as of March 31, 2010 was approximately $(9,340,000), or $(1.22) per share. Pro forma net tangible book value per share is determined by dividing (x) our total tangible assets less total liabilities by (y) the actual number of outstanding shares of our common stock plus the number of shares issuable upon conversion of all of our outstanding shares of preferred stock and bridge notes into common stock as if such conversion had occurred on March 31, 2010 after giving effect to a 1-for-4 reverse stock split as if it had occurred prior to March 31, 2010.
After giving effect to the sale of common stock offered in this offering at the assumed public offering price of $10.00 per share, and after deducting the estimated underwriting discounts and commissions and estimated offering expenses payable by us, our pro forma as adjusted net tangible book value as of March 31, 2010 would have been approximately $11,979,375, or $1.18 per share of common stock. This represents an immediate increase in pro forma as adjusted net tangible book value of $2.41 per share to existing stockholders and an immediate dilution of $8.82 per share to new investors purchasing our common stock in this offering. The following table illustrates this per share dilution to the new investors:
| Historical net tangible book value per share as of March 31, 2010 |
$ | (2.46 | ) | |
| Assumed initial public offering price |
10.00 | |||
| Pro forma net tangible book value per share as of March 31, 2010 |
(1.22 | ) | ||
| Increase in pro forma net tangible book value per share attributable to this offering |
2.41 | |||
| Pro forma as adjusted net tangible book value per share after offering |
1.18 | |||
| Dilution per share to new investors in this offering |
(8.82 | ) |
The following table summarizes, on a pro forma as adjusted basis as of March 31, 2010, the differences between the number of shares of common stock purchased from us, the total consideration and the average price per share paid by existing stockholders and by the investors participating in this offering, before deducting estimated underwriting discounts and commissions and estimated offering expenses payable by us, at an assumed initial public offering price of $10.00 per share, which is the mid-point of the range listed on the cover of this prospectus:
| Shares Purchased | Total Consideration | Average Price Per Share | ||||||||||||
| Number | Percent | Amount | Percent | |||||||||||
| Existing stockholders |
7,629,405 | 75.3 | % | $ | 37,574,000 | 60.0 | % | $ | 4.92 | |||||
| New investors |
2,500,000 | 24.7 | 25,000,000 | 40.0 | 10.00 | |||||||||
| Total |
10,129,405 | 100.0 | % | $ | 62,574,000 | 100.0 | % | $ | 6.18 | |||||
The number of shares of common stock outstanding in the table above is based on the pro forma number of shares outstanding as of March 31, 2010 which includes the assumed conversion of all of our outstanding shares of preferred stock and bridge notes into common stock, and assumes no exercise of the underwriters’over-allotment option. If the underwriters’ over-allotment option is exercised in full, the number of shares of common stock held by existing stockholders will be reduced to 72.6% of the total number of shares of common stock to be outstanding after this offering, and the number of shares of common stock held by investors participating in this offering will be increased to 2,875,000 shares or 27.4% of the total number of shares of common stock to be outstanding after this offering.
39
The above discussion and tables also assume no exercise of any outstanding stock options or warrants and no conversion of certain convertible promissory notes. As of March 31, 2010, there were:
| • | 600,625 shares of common stock issuable upon exercise of options issued under our stock option plans, at a weighted average exercise price of $3.61 per share; |
| • | 66,652 shares of common stock issuable upon exercise of options not issued under our stock option plans, at an exercise price of $9.64 per share; |
| • | 410,542 shares of common stock issuable upon exercise of warrants, at a weighted average exercise price of $3.48 per share; |
| • | 455,034 shares of common stock issuable upon the conversion of $3,641,667 in principal amount of, and interest on, convertible promissory notes, at a conversion price of $8.00 per share; and |
| • | 25,444 shares of common stock that may be issued pursuant to the placement agent warrant, at an exercise price of $8.00 per share, assuming an initial public offering price of $10.00 per share, which is the mid-point of the range listed on the cover of this prospectus. |
The following table summarizes, as of March 31, 2010 , after giving effect to the exercise of all stock options, warrants and convertible promissory notes outstanding as of March 31, 2010 as well as the assumed conversion of all of our outstanding shares of preferred stock and bridge notes into common stock, the differences between the number of shares of common stock purchased from us, the total consideration and the weighted average price per share paid by existing stockholders and by investors participating in this offering at an assumed initial public offering price of $10.00 per share, which is the mid-point of the range listed on the cover of this prospectus, before deducting underwriting discounts and commissions and estimated offering expenses:
| Shares Purchased | Total Consideration | Average Price Per Share | ||||||||||||
| Number | Percent | Amount | Percent | |||||||||||
| Existing stockholders |
9,187,702 | 78.6 | % | $ | 45,656,103 | 64.6 | % | $ | 4.97 | |||||
| New investors |
2,500,000 | 21.4 | 25,000,000 | 35.4 | 10.00 | |||||||||
| Total |
11,687,702 | 100.0 | % | $ | 70,656,103 | 100.0 | % | $ | 6.05 | |||||
Subsequent to March 31, 2010, we adopted our 2010 Incentive Compensation Plan, under which an aggregate of 1,250,000 shares of our common stock are reserved for issuance. To the extent that any outstanding options or warrants are exercised, new options are issued and those options are exercised or we issue additional shares of common stock in the future, there may be further dilution to investors participating in this offering.
40
We have derived the following statement of operations data for the years ended December 31, 2009, 2008 and 2007 and balance sheet data as of December 31, 2009 and 2008 from our audited financial statements included elsewhere in this prospectus. We have derived the following statement of operations data for the year ended December 31, 2006 and balance sheet data as of December 31, 2007 and 2006 from our audited financial statements not included in this prospectus. We have derived the following statement of operations data for the year ended December 31, 2005 and the balance sheet data as of December 31, 2005 from our unaudited financial statements not included in this prospectus. We derived the following selected historical financial data as of and for the three months ended March 31, 2010 and for the three months ended March 31, 2009 from our unaudited historical financial statements and the notes thereto included elsewhere in this prospectus. In the opinion of management, the interim financial data set forth below include all adjustments, consisting of normal recurring accruals, necessary to present fairly our financial position. Operating results for the three months ended March 31, 2010 are not necessarily indicative of the results that may be expected for the entire fiscal year. You should read the financial data set forth below in conjunction with our financial statements and related notes and the information under “Management’s Discussion and Analysis of Financial Condition and Results of Operations.” Our historical results are not necessarily indicative of our results to be expected in any future period.
| Three Months Ended March 31, |
Years Ended December 31, | |||||||||||||||||||||||||||
| 2010 | 2009 | 2009 | 2008 | 2007 | 2006 | 2005 | ||||||||||||||||||||||
| Statement of Operations Data: |
||||||||||||||||||||||||||||
| Related party license revenue |
$ | 650,000 | $ | 650,000 | $ | 2,600,000 | $ | 1,950,000 | $ | — | $ | — | $ | — | ||||||||||||||
| Operating costs and expenses: |
||||||||||||||||||||||||||||
| Research and development costs |
1,747,395 | 1,501,555 | 6,067,617 | 4,258,492 | 2,098,672 | 620,297 | 288,784 | |||||||||||||||||||||
| General and administrative expenses |
1,011,747 | 605,683 | 3,595,917 | 2,920,311 | 1,413,369 | 525,323 | 934,395 | |||||||||||||||||||||
| Gain on settlement of accounts payable |
— | — | — | — | — | (483,917 | ) | — | ||||||||||||||||||||
| Total operating expenses and costs |
2,759,142 | 2,107,238 | 9,663,534 | 7,178,803 | 3,512,041 | 661,703 | 1,223,179 | |||||||||||||||||||||
| Other (income) expense: |
||||||||||||||||||||||||||||
| Gain on debt extinguishment |
— | — | — | — | — | — | (301,309 | ) | ||||||||||||||||||||
| Loss on change in fair value of derivative liability |
209,350 | — | — | — | — | — | — | |||||||||||||||||||||
| Interest (income) expense, net |
197,220 | (32,325 | ) | 46,276 | 200,982 | 185,096 | 132,847 | 29,659 | ||||||||||||||||||||
| Loss before taxes |
(2,515,712 | ) | (1,424,913 | ) | (7,109,810 | ) | (5,429,785 | ) | (3,697,137 | ) | (794,550 | ) | (951,529 | ) | ||||||||||||||
| Income tax expense |
— | — | 49,250 | — | — | — | — | |||||||||||||||||||||
| Net loss |
$ | (2,515,712 | ) | $ | (1,424,913 | ) | $ | (7,159,060 | ) | $ | (5,429,785 | ) | $ | (3,697,137 | ) | $ | (794,550 | ) | $ | (951,529 | ) | |||||||
| Net loss per share (basic and diluted) |
$ | (0.49 | ) | $ | (0.27 | ) | $ | (1.34 | ) | $ | (1.04 | ) | $ | (0.74 | ) | $ | (0.16 | ) | $ | (0.20 | ) | |||||||
| Weighted average shares outstanding (basic and diluted) |
5,129,280 | 5,368,444 | 5,336,633 | 5,245,081 | 5,024,515 | 4,891,745 | 4,864,735 | |||||||||||||||||||||
| As of March 31, | As of December 31, | |||||||||||||||||||||||
| 2010 | 2009 | 2008 | 2007 | 2006 | 2005 | |||||||||||||||||||
| Balance Sheet Data: |
||||||||||||||||||||||||
| Cash and cash equivalents |
$ | 3,548,719 | $ | 2,569,129 | $ | 9,920,801 | $ | 3,611,814 | $ | 6,068,413 | $ | 57,026 | ||||||||||||
| Total assets |
6,574,176 | 4,673,688 | 10,955,360 | 3,730,092 | 6,109,753 | 130,393 | ||||||||||||||||||
| Deferred revenue |
8,546,374 | 9,196,374 | 11,685,099 | — | — | — | ||||||||||||||||||
| Convertible notes, net of discounts of $1,877,444 at March 31, 2010 and $1,129,000 at December 31, 2009 |
5,963,556 | 2,371,000 | — | — | — | — | ||||||||||||||||||
| Convertible preferred stock |
7,965,000 | 7,965,000 | 7,965,000 | 7,965,000 | 7,965,000 | — | ||||||||||||||||||
| Common stock |
54,551 | 54,551 | 54,518 | 50,338 | 49,695 | 48,307 | ||||||||||||||||||
| Additional paid-in capital |
26,803,455 | 25,794,862 | 25,653,645 | 24,039,925 | 23,172,908 | 23,510,487 | ||||||||||||||||||
| Treasury stock |
(1,679,234 | ) | (1,679,234 | ) | — | — | — | — | ||||||||||||||||
| Notes and subscriptions receivable, stockholder |
— | — | (573,620 | ) | (551,961 | ) | (630,361 | ) | (508,761 | ) | ||||||||||||||
| Accumulated deficit |
(44,538,676 | ) | (42,022,964 | ) | (34,863,904 | ) | (29,434,119 | ) | (25,736,982 | ) | (24,942,432 | ) | ||||||||||||
| Total stockholders’ equity (deficit) |
(11,394,904 | ) | (9,887,785 | ) | (1,764,361 | ) | 2,069,183 | 4,820,260 | (1,892,399 | ) | ||||||||||||||
41
MANAGEMENT’S DISCUSSION AND ANALYSIS OF
FINANCIAL CONDITION AND RESULTS OF OPERATIONS
The following discussion and analysis of our financial condition and results of operations should be read together with our financial statements and related notes included elsewhere in this prospectus. This discussion and analysis contains forward-looking statements that are based upon current expectations and involve risks, assumptions and uncertainties. You should review the “Risk Factors” section beginning on page 7 of this prospectus for a discussion of important factors that could cause actual results to differ materially from the results described in or implied by the forward-looking statements described in the following discussion and analysis.
Overview
We are a medical device company focused on the development and commercialization of technology that enables physicians to see inside the brain and heart using direct, intra-procedural MRI guidance while performing minimally invasive surgical procedures. Utilizing hospitals’ existing MRI suites, we believe that our marketed products and our product candidates will deliver better patient outcomes in shorter procedure times, enhance revenue potential for both physicians and hospitals, and reduce costs to the healthcare system. Our ClearPoint system is designed to allow minimally invasive procedures in the brain to be performed in the hospital’s MRI suite. The ClearTrace system is designed to allow catheter-based minimally invasive procedures in the heart to be performed in the hospital’s MRI suite. Finally, under our SafeLead Development Program, we are working with Boston Scientific to incorporate our MRI-safety technologies into Boston Scientific’s implantable leads for cardiac and neurological applications.
On June 16, 2010 we received regulatory clearance from the FDA to market our ClearPoint system in the United States for general neurological procedures. As such, our financial statements do not reflect any revenues generated from sales of our ClearPoint system products. We do not have regulatory clearance or approval to sell the ClearTrace system and, therefore, we have not generated revenues from sales of that product candidate. In 2008, we received licensing fees totaling $13,000,000 from Boston Scientific for our MRI-safety technologies, which we used to finance our operations and internal growth. We have also financed our operations and internal growth through private placements of securities, borrowings and interest earned on the net proceeds from our private placements and the Boston Scientific licensing fees. Prior to 2008, we were a development stage enterprise. We have incurred significant losses since our inception in 1998 as we have devoted substantially all of our efforts to research and development. As of March 31, 2010, we had an accumulated deficit of approximately $44,539,000. We may continue to incur significant operating losses as we commercialize our marketed products, continue to develop our product candidates and expand our business generally. We also expect that our general and administrative expenses will increase due to additional operational and regulatory costs and burdens associated with operating as a public company.
Recent Developments
No material development has occurred since the conclusion of our quarterly period ended March 31, 2010 with the exception of our receipt of FDA clearance to market our ClearPoint system, which is discussed elsewhere in this prospectus.
Factors Which May Influence Future Results of Operations
The following is a description of factors which may influence our future results of operations, including significant trends and challenges that we believe are important to an understanding of our business and results of operations.
Revenues
Since inception, we have generated revenues primarily from our collaborative agreements with Boston Scientific, principally from recognition of portions of the $13,000,000 of licensing fees, which we received in
42
2008. Revenues associated with these licensing fees are recognized on a straight-line basis over a five year period, which is our estimated period of continuing involvement in the development activities. Additional payments related to substantive, performance-based milestones and incentive payments that may be received under the agreement regarding implantable cardiac leads will be deferred upon receipt and achievement of the specified milestones and recognized over our estimated period of continuing involvement. These revenue recognition policies are more fully described in the “Critical Accounting Policies and Significant Judgments and Estimates” section below. We did not report any revenues in 2007, 2006 or 2005.
On June 16, 2010 we received 510(k) clearance from the FDA to market our ClearPoint system in the United States for general neurological procedures. Future revenues from sales of our marketed products are difficult to predict and may not be sufficient to offset our continuing and increasing research and development expenses and selling, general and administrative expenses for the next several years. We cannot sell any of our product candidates until we receive regulatory clearance or approval.
The generation of recurring revenues through sales of our disposable components is an important part of our business model for our ClearPoint system. We anticipate that recurring revenues will constitute an increasing percentage of our total revenues as we leverage each new installation of our ClearPoint system to generate recurring sales of these disposable components. With respect to a single hospital, we do not anticipate that sales of the reusable components of our ClearPoint system will generate recurring revenues.
Research and Development Expenses
Our research and development expenses consist primarily of costs associated with the conceptualization, design, testing and prototyping of our ClearPoint system products and our product candidates. This includes: the salaries, travel and benefits of research and development personnel; materials and laboratory supplies used by our research personnel; consultant costs; sponsored contract research and product development with third parties; and licensing costs. From our inception through March 31, 2010, we have incurred approximately $26,983,000 in research and development expenses. We anticipate that research and development expenses will increase as we: (i) continue to develop enhancements to our ClearPoint system; (ii) continue our early-stage product development efforts for the ClearTrace system; (iii) commence clinical trials for the ablation catheter component of the ClearTrace system; and (iv) expand our research to apply our technologies to additional product applications.
Product development timelines, likelihood of success and total costs vary widely by product candidate. At this time, due to the risks inherent in the product clearance and approval process and given the early stage of development of our product candidates, we are unable to estimate with any certainty the costs that we will incur in the continued development of our product candidates for commercialization.
General and Administrative Expenses
Our general and administrative expenses consist primarily of: salaries, travel and benefits for administrative personnel; share-based compensation; professional fees, including fees for attorneys and outside accountants; selling costs; occupancy costs; insurance; and other general and administrative expenses, which include corporate licenses and taxes, postage, office supplies and meeting costs. Our general and administrative expenses are expected to increase due to costs associated with the commercialization of our ClearPoint system, increased headcount necessary to support our continued growth in operations, and the additional operational and regulatory burdens and costs associated with operating as a publicly traded company. In addition, we expect to incur additional costs associated with protecting our intellectual property rights as necessary to support our product offerings.
Critical Accounting Policies and Significant Judgments and Estimates
Our management’s discussion and analysis of our financial condition and results of operations are based on our financial statements, which have been prepared in accordance with accounting principles generally accepted in the United States, or GAAP. The preparation of these financial statements requires us to make estimates and
43
assumptions that affect the reported amounts of assets and liabilities and the disclosure of contingent assets and liabilities as of the date of the financial statements as well as the reported expenses during the reporting periods. The accounting estimates that require our most significant, difficult and subjective judgments include revenue recognition, impairment of long-lived assets, computing the fair value of our derivative liability and the determination of share-based compensation. We evaluate our estimates and judgments on an ongoing basis. Actual results may differ materially from these estimates under different assumptions or conditions.
While our significant accounting policies are more fully described in note 2 to our financial statements included elsewhere in this prospectus, we believe that the following accounting policies and estimates are most critical to a full understanding and evaluation of our reported financial results.
Revenue Recognition. We evaluate revenue recognition on an agreement-by-agreement basis which, as of March 31, 2010, principally involved two license agreements with Boston Scientific. Both agreements provide various revenue streams for us, including an up-front licensing fee for one of the licenses, various milestone payments, payments for research and development and consulting services, and royalties.
We evaluate the various elements of these agreements based upon GAAP for multiple element arrangements to determine whether the various elements represent separate units of accounting. This evaluation requires subjective determinations about the fair value of each element and whether delivered elements have stand alone value and, therefore, are separable from the undelivered contract elements for revenue recognition purposes. In addition, we evaluated repayment provisions associated with one of the license agreements which, under certain conditions, would require us to return payments received under the agreement. In both license agreements, we concluded that all of the contract elements should be treated as a single unit of accounting. As such, all amounts received were initially recorded as deferred revenue and thereafter recognized as revenue over our estimated period of performance on a straight-line basis. In the case of the license with possible repayment obligation provisions, revenue recognition will not occur until the repayment obligation period expires.
Note 2 to our financial statements, “Significant Accounting Policies—Revenue Recognition”, more fully describes the deliverables under these license agreements including our rights, obligations and cash flows.
Inventory. Inventory is carried at the lower of cost (first-in, first-out method) or net realizable value for products that are cleared or approved for commercial sale or for which clearance or approval is anticipated. As of March 31, 2010, all items included in inventory related to our ClearPoint system. At each reporting period in which our balance sheet reflects inventory related to product candidates that do not have regulatory clearance or approval, we evaluate the likelihood of receiving regulatory clearance or approval for these product candidates based on input from our external regulatory advisers. We also consider our anticipated selling prices based on analysis of product pricing of competitors and review of market information prepared by third party research analysts to determine net realizable value.
Valuation Allowance for Deferred Tax Assets and Liabilities. Deferred tax assets and liabilities are recognized for the estimated future tax consequences attributable to differences between the financial statement carrying amounts of existing assets and liabilities and their respective income tax bases. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. The effect on deferred tax assets and liabilities of a change in tax rates is recognized in the period that included the enactment date.
Valuation allowances are recorded for deferred tax assets when the recoverability of such assets is not deemed more likely than not.
We have evaluated the effect of the guidance provided by GAAP regarding accounting for uncertainty in income taxes that became effective in 2009. In that regard, we have evaluated all tax positions that could have a significant effect on the financial statements and determined we have no uncertain tax positions at March 31, 2010 that could have a significant effect on our financial statements. Our tax returns after 2005 remain open for examination.
44
Impairment of long-lived assets. We evaluate the recoverability of our long-lived assets (finite lived intangible assets and property and equipment) whenever events or changes in circumstances indicate that the carrying amount of long-lived assets may not be fully recoverable. When this occurs, the expected undiscounted future cash flows are compared to the net book value of the related assets. If the net book value of the related assets exceeds the expected undiscounted future cash flows of the assets, the carrying amount will be reduced to the present value of the expected future cash flows and an impairment loss would be recognized.
Share-based compensation. We account for compensation for all arrangements under which employees and others receive shares of stock or equity instruments (including options and warrants) in accordance with FASB ASC Topic 718 “Compensation – Stock Compensation”, or ASC Topic 718. Under ASC Topic 718, the fair value of each award is estimated and amortized as compensation expense over the requisite service period. The fair value of our share-based awards is estimated on the grant date using the Black-Scholes valuation model. This valuation model requires the input of highly subjective assumptions, including the expected price volatility and estimated option term. As we have been operating as a private company, we are unable to use actual price volatility and option life data as input assumptions within our Black-Scholes valuation model. Prior to October 2009, we used expected volatilities based on the historical volatility of the industry sector in which we operate, in accordance with the guidance set forth in ASC Topic 718.
Beginning in October 2009, we based our estimate of expected volatility on the average historical volatilities of publicly traded companies we deemed similar due to our lack of historical volatility data of our own. We will consistently apply this methodology until a sufficient amount of historical information regarding the volatility of our share price becomes available.
To estimate the expected term, we chose to utilize the “simplified” method for “plain vanilla” options as discussed in the Securities and Exchange Commission’s Staff Accounting Bulletin 107, or SAB 107. We believe that all factors listed in SAB 107 as pre-requisites for utilizing the simplified method are true for us and for our share-based payment arrangements. We intend to utilize the simplified method for the foreseeable future until more detailed information about exercise behavior becomes available.
Our risk-free interest rates are based on a zero-coupon U.S. treasury instrument, the term of which is consistent with the expected term of the stock options. We have not paid and do not anticipate paying cash dividends on our shares of common stock; therefore, the expected dividend yield is assumed to be zero. The fair value of share-based payments are generally amortized on a straight-line basis over the requisite service periods of the awards, which are generally the vesting periods.
We believe there is a high degree of subjectivity involved when using option pricing models to estimate share-based compensation under ASC Topic 718. Currently, there is not a market-based mechanism or other practical application to verify the reliability and accuracy of the estimates stemming from these valuation models, nor is there a means to compare and adjust the estimates to actual values. Although the fair value of stock option awards is determined in accordance with ASC Topic 718 using an option pricing model, that value may not be indicative of the fair value observed in a market transaction between a willing buyer and a willing seller. If factors change and we employ different assumptions in the application of ASC Topic 718 in future periods than those currently applied under ASC Topic 718, the compensation expense we record in future periods under ASC Topic 718 may differ significantly from what we have historically reported.
Total share-based compensation expense for the three months ended March 31, 2010 and 2009 was approximately $54,000 and $38,000, respectively, and for years ended December 31, 2009 and 2008, it was approximately $131,000 and $118,000, respectively. As of March 31, 2010 there was approximately $343,000 of unrecognized compensation cost related to nonvested share-based compensation arrangements. That cost is expected to be recognized over a weighted-average period of approximately three years.
Research and development costs. Research and development costs consist of direct and indirect costs associated with the development of our technologies. These costs are expensed as incurred.
45
Derivative Financial Instruments. We account for derivative instruments in accordance with FASB ASC Topic 815, which establishes accounting and reporting standards for derivative instruments and hedging activities, including certain derivative instruments embedded in other financial instruments or contracts and requires recognition of all derivatives on the balance sheet at fair value. We calculate the fair value of these instruments using the Black-Scholes valuation model. Changes in the fair value of derivatives are recorded each period as a gain or loss in the statement of operations unless the derivative qualifies for hedge accounting. At March 31, 2010 and at December 31, 2009 and 2008, we did not have any derivative instruments that were designated as hedges.
Results of Operations
Comparison of the Three Months Ended March 31, 2010 to the Three Months Ended March 31, 2009
| March 31, | Percentage Change | ||||||||
| 2010 | 2009 | ||||||||
| Revenues |
$ | 650,000 | $ | 650,000 | 0% | ||||
| Research and development costs |
1,747,000 | 1,502,000 | 16 | ||||||
| General and administrative expenses |
1,012,000 | 606,000 | 67 | ||||||
| Other (income) expense, net |
407,000 | (32,000 | ) | — | |||||
Revenues. Revenues were $650,000 for both the three months ended March 31, 2010 and 2009. Revenues for both periods relate solely to our licensing and development agreements with Boston Scientific.
Research and development costs. Research and development cost was approximately $1,747,000 for the three months ended March 31, 2010, compared to approximately $1,502,000 for the three months ended March 31, 2009, an increase of approximately 16%. This increase was due mostly to an increase of approximately $219,000 in third party research and development related services and an increase in regulatory filing costs related to our ClearPoint system of approximately $104,000. These increases were partially offset by a decrease in materials and supplies for product candidate testing and prototyping of approximately $68,000 related primarily to the timing of development activities associated with our ClearPoint system.
General and administrative expenses. General and administrative expenses were approximately $1,012,000 for the three months ended March 31, 2010, compared to approximately $606,000 for the three months ended March 31, 2009, an increase of approximately 67%. The increase was due primarily to: (i) an increase of approximately $170,000 in patent filing and prosecution costs related primarily to intellectual property associated with our ClearPoint system; (ii) an increase of approximately $112,000 related to accruals for annual bonuses; and (iii) an increase of approximately $77,000 in sales and marketing costs incurred in preparation of the anticipated commercial launch of our ClearPoint system.
Other (income) expense, net. Net other expense was approximately $407,000 for the three months ended March 31, 2010, compared to other income of approximately $32,000 for the three months ended March 31, 2009. A loss of approximately $209,000 related to the change in fair value of our derivative liability was recorded during the three months ended March 31, 2010. Interest expense for the three months ended March 31, 2010 was approximately $201,000, while no interest expense was incurred during the same period in 2009. Interest expense of approximately $153,000 relates to the accretion of a discount and accrued interest on related party convertible notes payable with a principal balance of $3,500,000. All other interest expense relates to the bridge notes that were issued in March 2010. Interest income for the three months ended March 31, 2010 decreased by approximately $29,000 compared to the same period in 2009 due to lower average cash balances and lower interest rates.
46
Comparison of the Year Ended December 31, 2009 to the Year Ended December 31, 2008
| December 31, | Percentage Change |
||||||||
| 2009 | 2008 | ||||||||
| Revenues |
$ | 2,600,000 | $ | 1,950,000 | 33 | % | |||
| Research and development costs |
6,068,000 | 4,258,000 | 43 | ||||||
| General and administrative expenses |
3,596,000 | 2,920,000 | 23 | ||||||
| Interest expense, net |
46,000 | 201,000 | (77 | ) | |||||
Revenues. Revenues were approximately $2,600,000 for the year ended December 31, 2009 compared to approximately $1,950,000 for the year ended December 31, 2008, an increase of approximately 33%. Revenues for both periods relate solely to our licensing and development agreements with Boston Scientific. The increase in revenues resulted from the recognition of a full twelve months of licensing fee revenues during the year ended December 31, 2009 compared to the recognition of only nine months of licensing fee revenues for the year ended December 31, 2008.
Research and development costs. Research and development cost was approximately $6,068,000 for the year ended December 31, 2009, compared to approximately $4,258,000 for the year ended December 31, 2008, an increase of approximately 43%. This increase was due primarily to: (i) an increase of approximately $1,177,000 related to the employment of additional research and development personnel; (ii) an increase of approximately $746,000 related to the use of third-parties for research and development services; and (iii) an increase of approximately $441,000 for materials and supplies necessary for product candidate testing and prototyping, depreciation and miscellaneous research and development expenses. The increase in research and development expenses was offset by decreases in engineering design costs and software development costs of approximately $356,000 and $301,000, respectively.
General and administrative expenses. General and administrative expense was approximately $3,596,000 for the year ended December 31, 2009 compared to approximately $2,920,000 for the year ended December 31, 2008, an increase of approximately 23%. The increase was due primarily to: (i) an increase of approximately $527,000 in corporate and operations personnel costs; (ii) an increase of approximately $263,000 in sales and marketing costs incurred in preparation of the anticipated commercial launch of our ClearPoint system; and (iii) an increase of approximately $129,000 in occupancy costs. Increases in corporate and operating personnel costs were caused mostly by additional hires. The increase in occupancy costs was associated with a full year of lease expense for the year ended December 31, 2009 for both our Irvine, California and Memphis, Tennessee offices as compared to only occupying these offices a portion of the year during 2008. Increases in general and administrative expenses were partially offset by an approximate $360,000 reduction in professional fees during the year ended December 31, 2009 related to the timing of patent filings.
Interest expense, net. Net interest expense was approximately $46,000 for the year ended December 31, 2009 compared to net interest expense of approximately $201,000 for the year ended December 31, 2008, a decrease of approximately 77%. Interest expense decreased for the year ended December 31, 2009 as compared to the year ended December 31, 2008 as a result of the amortization of a debt discount related to a convertible note converted in June 2008 of approximately $395,000 as compared to amortization of debt discount of approximately $98,000 since inception of the related party convertible note payable in October 2009 through December 31, 2009. Interest income in 2009 decreased from 2008 by approximately $50,000 due to lower average cash balances.
47
Comparison of the Year Ended December 31, 2008 to the Year Ended December 31, 2007
| December 31, | Percentage Change |
||||||||
| 2008 | 2007 | ||||||||
| Revenues |
$ | 1,950,000 | $ | — | — | ||||
| Research and development costs |
4,258,000 | 2,099,000 | 103 | % | |||||
| General and administrative expenses |
2,920,000 | 1,413,000 | 107 | ||||||
| Interest expense, net |
201,000 | 185,000 | 9 | ||||||
Revenues. Revenues for the year ended December 31, 2008 were approximately $1,950,000, compared to zero for the year ended December 31, 2007. The increase in revenues resulted primarily from the recognition of revenues from the licensing fees received under one of our agreements with Boston Scientific.
Research and development costs. Research and development cost for the year ended December 31, 2008 was approximately $4,258,000, compared to $2,099,000 for the year ended December 31, 2007, an increase of approximately 103%. This increase was due primarily to: (i) an increase of approximately $474,000 related to the employment of additional research and development personnel; (ii) an increase of approximately $959,000 related to engineering, design and documentation, materials, third party contract research associated with the development of our ClearPoint system; (iii) an increase of approximately $263,000 related to sponsored research programs; (iv) an increase of $313,000 in payments related to the acquisition of licenses; and (v) an increase of approximately $119,000 related to the use of consultants.
General and administrative expenses. General and administrative expense for the year ended December 31, 2008 was approximately $2,920,000 compared to $1,413,000 for the year ended December 31, 2007, an increase of 107%. This increase was due primarily to: (i) an increase of approximately $540,000 in corporate personnel expense relating to the employment of additional administrative personnel; (ii) an increase of approximately $110,000 in occupancy expense primarily related to rent expense and leasehold improvements at our facility in Irvine, California; (iii) an increase of approximately $690,000 in professional fees, primarily legal fees, incurred for patent costs; (iv) an increase of approximately $94,000 in travel related costs; and (v) an increase of approximately $58,000 related to depreciation expense on property additions.
Interest expense, net. Net interest expense for the year ended December 31, 2008 was approximately $201,000 compared to $185,000 for the year ended December 31, 2007, an increase of approximately 9%. The difference in net interest expense is a result of the change in the amount of interest income we earned. Although our average cash balances in 2008 were higher than that of 2007, the decline in rate of interest that we earned on our cash balances decreased significantly as we focused on preservation and safeguarding of cash rather than maximizing interest income, resulting in approximately $16,000 less in interest income in 2008 as compared to 2007. The interest expense for both 2007 and 2008 were the same amount, approximately $395,000, representing the charge to interest expense for the amortization of the value of warrants granted in connection with a note to Boston Scientific. The value assigned to the warrants was recorded as a discount to the note at the time of issuance and was amortized over the period of time that the note was outstanding.
Determination of Fair Market Value of Our Common Stock
The exercise prices of options granted were set by our Board of Directors. Our Board of Directors sets the exercise prices of options based on its determination of the fair market value of our common stock at the time of the grants, which determination is made in accordance with federal tax rules, which require reasonable application of a reasonable valuation method.
48
We performed valuations of our common stock contemporaneously with the granting of stock options. We believe that all of our stock options have been granted with exercise prices that are equal to or greater than the fair market value of our common stock on the date of grant. From January 1, 2009 through March 31, 2010, we have granted the following compensatory stock options:
| Grant date |
Number of Options |
Exercise Price |
Fair Value of Common Stock(1) |
Fair Value of Option Grant(2) |
Intrinsic Value(3) |
|||||||||
| March 17, 2009 |
2,500 | $ | 9.64 | $ | 9.64 | $ | 2.68 | $ | 900 | |||||
| August 20, 2009 |
9,250 | 9.64 | 9.64 | 2.68 | 3,330 | |||||||||
| December 10, 2009 |
15,000 | 9.64 | 9.64 | 3.24 | 5,400 | |||||||||
| December 22, 2009 |
66,652 | 9.64 | 9.64 | 2.76 | 23,995 | |||||||||
| (1) | All fair market valuations were determined by our Board of Directors in consultation with management at the date of each stock option grant. |
| (2) | As determined using the Black-Scholes valuation model at the date of each stock option grant. |
| (3) | Intrinsic value reflects the amount by which $10.00, which is the mid-point of the range listed on the cover of this prospectus, exceeds the exercise price of the outstanding stock options. |
At March 31, 2010, we had 667,277 compensatory stock options outstanding with an intrinsic value of $3,862,820. Intrinsic value reflects the amount by which $10.00, which is the mid-point of the range listed on the cover of this prospectus, exceeds the exercise price of the outstanding stock options.
Significant factors, assumptions and methodologies used in determining fair value of our common stock on the grant dates of stock option awards made subsequent to January 1, 2009
We granted compensatory stock options on four dates between January 1, 2009 and March 31, 2010. In the absence of a public trading market for our common stock, we determined a reasonable estimate of the then current fair value of our common stock based upon multiple valuation criteria and contemporaneous analyses. Our Board of Directors exercised judgment in evaluating and assessing the fair value of our common stock on each grant date. Set forth below are significant factors considered, assumptions made and methodologies used in determining fair value on each grant date.
Valuation Methodologies for March 17, 2009 and August 20, 2009 Grants
General. The fair values for the March 17, 2009 and August 20, 2009 grants each utilized, in part, two alternative valuation approaches. The first approach, referred to as the income approach, is a valuation technique that provides an estimation of the fair value of a business based upon the cash flows that it can be expected to generate over time. The second approach, referred to as the market approach, is a valuation technique that provides an estimation of fair value based on recent transactions that have occurred in our stock, our industry or in related industries.
Income Approach. The income approach we utilized begins with an estimation of the annual cash flows that a business is expected to generate over a discrete projection period. The estimated cash flows for each of the years in the period are then converted to their present value equivalent using a discount rate considered appropriate given the risk of achieving the projected cash flows. We selected a discount rate of 35%. In selecting the discount rate, we looked at what we believe are current rates of return expected by investors in various investments with different risk characteristics and we established the discount rate based on what we believed to be the most comparable rate of return range. The present value of the estimated cash flows are then added to the present value equivalent of the residual value of the business at the end of the projection period to arrive at an estimate of fair value. Such an approach necessarily relies on estimations of future cash flows that are inherently uncertain, as well as a determination of an appropriate discount rate in order to derive present value equivalents of both the projected cash flows and the residual value of the business at the end of the period. The use of different estimations of future cash flows or a different rate of return could result in a different indication of fair value.
49
Market Approach. The market approach utilized recent sales of our common stock in privately negotiated transactions between our stockholders.
Determination of Value. In determining a value, we considered the indications of value from both the income approach and the market approach.
| • | Weighting. Application of the market approach resulted in a higher indication of value than the income approach. We applied a 20% weighting to the income approach and an 80% weighting to the market approach in deriving a final indication of value. We chose to apply only a 20% weighting to the income approach because the assumptions underlying the income approach are subject to significant volatility. We chose to apply an 80% weighting to the market approach because of the isolated and limited nature of the private transactions in our stock. |
| • | Discount for Lack of Control. The discount for lack of control, otherwise known as a discount for minority interest, reflects a reduction in value due to the absence of elements of control that do not accrue to a minority stockholder. We selected a lack of control discount of 30%, based on a review of premiums paid in transactions to acquire control of public companies that ranged from 20% to 50%. The lack of control discount took into account the rights, privileges and preferences held by our preferred stockholders. |
| • | Discount for Lack of Marketability. A discount for lack of marketability is applied to a minority interest in the equity of a privately-held company due to the fact that a stockholder in a privately-held company has no ready market for his or her interest other than by a private sale to another stockholder or willing buyer. We selected a lack of marketability discount of 35% based on restricted stock studies, studies of private placements of stock in public companies and studies of initial public offerings that primarily observed discounts ranging from 30% to 40%. We chose the mid-point of that range in valuing our common stock due to the historical lack of dividends being paid, restrictions on transferability, and the high volatility of our peer group. |
Fair Value at March 17, 2009. To determine the fair value of our common stock on March 17, 2009 of $9.64 per share, our primary considerations included:
| • | an August 21, 2008 valuation prepared by an unrelated valuation specialist utilizing the valuation methods described above; |
| • | our historical operating and financial results, current cash position and estimated time that our current cash position would fund our operations; |
| • | the liquidation preference and other rights, privileges and preferences associated with our preferred stock; |
| • | our stage of development and business strategy, including the status and estimated timing of clearance of our 510(k) submissions with the FDA for our ClearPoint system and the likelihood and timing of product launch; and |
| • | prevailing economic conditions and outlook at the time. |
Fair Value at August 20, 2009. To determine the fair value of our common stock on August 20, 2009 of $9.64 per share, our primary considerations included:
| • | the August 21, 2008 valuation and the other factors considered in the March 17, 2009 fair value determination noted above; |
| • | the declining cash balances of the company; |
| • | continued uncertainty regarding FDA clearance of the pending 510(k) submissions for our ClearPoint system; |
| • | the determination by our Board of Directors that no event had occurred which, in their judgment, resulted in either a higher or lower value of our common stock than the March 17, 2009 grants; and |
50
| • | the lack of free accessibility as of such date to the equity capital markets, creating difficulties in marketability and liquidity. |
Valuation Methodology for December 10, 2009 and December 22, 2009 Grants
General. Because we began the process of preparing for our initial public offering in the fourth quarter of 2009, we amended our process to estimate the value of our common stock to utilize a probability-weighted expected return method, or “PWER method”, as detailed in a practice aid issued by the American Institute of Certified Public Accountants entitled “Valuation of Privately Held Company Equity Securities Issued as Compensation”. A PWER method analysis consists of the following five steps:
| • | Identifying the most likely liquidity events for the company, including when they are expected to occur, the probability of each occurring, and the equity values of the company for each. Scenarios considered can be broken down into four general categories: a strategic sale or merger; an initial public offering; the dissolution of the company; and the company’s private enterprise value (with no liquidity event); |
| • | Determining the value of the common stock for, and as of, each of the liquidity events considered; |
| • | Determining the present value of the common stock for each liquidity scenario; |
| • | Applying the probabilities assigned to each scenario to the present value of the common stock for each scenario to determine the probability-weighted value as of the valuation date; and |
| • | Performing a check-to-value analysis to determine the reasonableness of the value of the common stock and the assumptions relied on. |
Using this valuation methodology, we estimated the value of our common stock based upon an analysis of future values of the company assuming various liquidity events as described below.
Identifying Most Likely Liquidity Events. We determined that there were four likely liquidity events:
| • | a sale of our intellectual property in a liquidation scenario; |
| • | completing an initial public offering of our common stock with FDA clearance for our ClearPoint system; |
| • | completing an initial public offering of our common stock without FDA clearance for our ClearPoint system; and |
| • | a sale of the company as a going concern. |
Determining the Value of Common Stock Under Each Liquidity Scenario. The value resulting from a sale of our intellectual property was based upon management’s expectations as of the valuation date. The value resulting from both initial public offering liquidity events was based upon preliminary discussions of value with the underwriter. The value resulting from a sale of the company was based on conversations with investment bankers, potential buyers and management’s expectations as of the valuation date.
Determining Present Value. We selected a discount rate of 35%. In selecting the discount rate, we looked at what we believe are current rates of return expected by investors in various investments with different risk characteristics and we established the discount rate based on what we believe to be the most comparable rate of return range.
Weighting of Each Scenario. The significant drivers and weightings for our December 10, 2009 and December 22, 2009 valuations were:
| • | sale of intellectual property in a liquidation scenario, 5%; |
| • | initial public offering with FDA clearance for our ClearPoint system, 75%; |
51
| • | initial public offering without FDA clearance for our ClearPoint system, 0%; and |
| • | sale of the company as a going concern, 20%. |
We assigned only a 5% weighting to a sale of intellectual property in a liquidation scenario because we viewed a liquidation sale as unlikely to occur. We assigned a 75% weighting to the initial public offering with FDA clearance for our ClearPoint system because we deemed that scenario to be the most likely liquidity event. We assigned a 0% weighting to an initial public offering without FDA clearance for our ClearPoint system because we believed we would have FDA clearance of our ClearPoint system prior to completing the initial public offering. We assigned a 20% weighting to a sale of the company, as we believed it was the second most likely liquidity event.
Discount for Lack of Control. The discount for lack of control, otherwise known as a discount for minority interest, reflects a reduction in value due to the absence of elements of control that do not accrue to minority stockholder. We selected a lack of control discount of 20%, based on a review of premiums paid in transactions to acquire control of public companies that ranged from 20% to 50%.
Discount for Lack of Marketability. A discount for lack of marketability is applied to a minority interest in the equity of a privately-held company due to the fact that a stockholder in a privately-held company has no ready market for his or her interest other than by a private sale to another stockholder or willing buyer. Restricted stock studies, studies of private placements of stock in public companies and studies of initial public offerings primarily observed discounts ranging from 30% to 40%. However, we selected a lack of marketability discount of 20% because we had commenced the initial public offering process.
Fair Value at December 10, 2009. To determine the fair value of our common stock on December 10, 2009 of $9.64 per share, our primary considerations included:
| • | a November 17, 2009 valuation prepared by an unrelated valuation specialist utilizing the PWER method described above; |
| • | the determination by our Board of Directors that no event had occurred which, in their judgment, resulted in either a higher or lower value of our common stock than the March 17, 2009 grants in 2009; |
| • | our historical operating and financial results, current cash position and estimated time that our current cash position would fund our operations; and |
| • | uncertainty regarding FDA clearance of the final 510(k) submission for our ClearPoint system. |
Fair Value at December 22, 2009. To determine the fair value of our common stock on December 22, 2009 of $9.64 per share, our primary considerations included:
| • | a December 21, 2009 valuation prepared by an unrelated valuation specialist utilizing the PWER method described above; |
| • | the determination by our Board of Directors that no event had occurred which, in their judgment, resulted in either a higher or lower value of our common stock than the March 17, 2009 grants in 2009; |
| • | our historical operating and financial results, current cash position and estimated time that our current cash position would fund our operations; and |
| • | uncertainty regarding FDA clearance of the final 510(k) submission for our ClearPoint system. |
Changes in Value of Our Common Stock Since December 22, 2009
As a result of the analysis conducted by us and the underwriters, we estimate that the initial public offering price of our common stock will be $10.00 per share, which is the mid-point of the range listed on the cover of this prospectus. The difference between the estimated fair value of our common stock of $9.64 per share for all
52
issuances of options between March 17, 2009 and December 22, 2009 and the initial public offering price takes into account several factors considered by our board of directors and the underwriters:
| • | our receipt of 510(k) marketing clearance from the FDA to market our ClearPoint system in the United States for general neurological interventional procedures; |
| • | an analysis of the typical valuation ranges seen in recent initial public offerings for companies in our industry with similar market capitalization; |
| • | a review of current market conditions and the results of operations, competitive position and the stock performance of our competitors; and |
| • | consideration of our history as a private company. |
Liquidity and Capital Resources
We received $13,000,000 in licensing fees in 2008 under one of our agreements with Boston Scientific. We recognize revenue from these licensing fees over the estimated time period to complete our development work under the agreement. In addition, we are entitled to receive up to $21,600,000 in future milestone-based payments, subject to our achievement of the milestones stipulated in the agreements and the issuance of certain patents licensed to Boston Scientific, of which there can be no assurance. In addition to payments received from Boston Scientific, we have financed our operations and internal growth almost exclusively through private placements of preferred stock and borrowings. We have incurred significant losses since our inception in 1998. As of March 31, 2010, we had an accumulated deficit of approximately $44,539,000. Our accumulated deficit resulted principally from research and development activities and the costs to support such efforts as recorded in general and administrative costs.
During 2009, Boston Scientific loaned us $3,500,000 pursuant to the terms of three convertible promissory notes. Interest on the loans accrues at 10% per annum and compounds annually. The Boston Scientific loans are secured by a first priority security interest in all of our assets. Each loan matures on the second anniversary of the date on which the funds were advanced. In addition, we will be required to prepay all or a portion of loans upon the consummation of any qualified financing, which is any equity financing in which shares of our preferred stock are issued in exchange for cash proceeds. Upon consummation of a qualified financing from Medtronic, Inc., St. Jude Medical, Inc., or Johnson & Johnson, or any of their respective subsidiaries or affiliates, up to 100% of the cash proceeds from such qualified financing must be used to prepay the outstanding principal of the loans and accrued interest thereon. Upon consummation of a qualified financing from any other investor, up to 25% of the cash proceeds from such qualified financing shall be applied by us to prepay the outstanding principal of the loans and accrued interest thereon. We can prepay each loan at any time prior to its respective maturity date. At the option of Boston Scientific, these loans are convertible into one share of our common stock for every $8.00 of principal and interest outstanding at the time of conversion. To the extent that Boston Scientific has not exercised its conversion right prior to the completion of this offering, Boston Scientific will no longer have the right to convert the notes into shares of stock.
In March 2010, we issued 10% senior unsecured convertible notes, or the bridge notes, in the aggregate principal amount of $4,071,000 in a private placement, or the bridge financing. Upon consummation of this offering, the bridge notes will automatically convert into shares of our common stock at the lesser of $8.00 per share or 80% of the public offering price, but the conversion price cannot be lower than $4.00 per share. The bridge notes mature two years from the date of issuance, unless earlier converted, and accrue interest at the rate of 10% per annum. All accrued interest will be paid in cash and will not be converted into shares of our common stock. We incurred approximately $293,000 of expenses related to the bridge financing, including legal fees and placement agent fees. In addition, we issued warrants to the placement agent exercisable for 25,444 shares of our common stock at a price equal to the lesser of $8.00 per share or 80% of the public offering price of our common stock in this offering, but the exercise price cannot be lower than $4.00 per share. We intend to use the proceeds of the bridge financing for working capital and general corporate purposes.
53
Net Cash Flows from Operating Activities. Net cash flows from operating activities for the three months ended March 31, 2010 and 2009 and the years ended December 31, 2009, 2008 and 2007 was approximately $(2,632,000), $(2,419,000), $(9,479,000), $7,256,000, and $(2,994,000), respectively. The use of cash in the three months ended March 31, 2010 and 2009 and the years ended December 31, 2009 and 2007 resulted primarily from funding research and development activities and from incurring supporting general and administrative expenses. The positive net cash for the year ended December 31, 2008 resulted from the $13,000,000 in licensing fees under one of our agreements with Boston Scientific.
Net Cash Flows from Investing Activities. Net cash flows from investing activities for the three months ended March 31, 2010 and 2009 and the years ended December 31, 2009, 2008 and 2007 was approximately $(33,000), $(154,000), $(282,000), $(947,000), and $(62,000), respectively. Net cash used in investing activities for each of the periods was primarily related to the purchase of property and equipment and the acquisition of intellectual property licenses.
Net Cash Flows from Financing Activities. Net cash flows from financing activities for the three months ended March 31, 2010 and 2009 and the years ended December 31, 2009, 2008 and 2007 was approximately $3,645,000, $(1,000,000), $2,409,000, zero, and $600,000, respectively. Net cash flows from financing activities for the year ended December 31, 2007 was attributable to borrowings under related party notes and the issuance of preferred stock. Net cash flows from financing activities for the year ended December 31, 2009 related primarily to the proceeds from our issuance of related party convertible notes payable, less the issuance of notes receivable and the purchase of treasury stock. Cash flows from financing activities for the three months ended March 31, 2010 related primarily to proceeds from our issuance of the bridge notes.
Operating Capital and Capital Expenditure Requirements. To date, we have not achieved profitability. We anticipate that we may continue to incur substantial net losses for the next several years as we commercialize our ClearPoint system products, continue to develop our product candidates, expand our corporate infrastructure and pursue additional applications for our technology platforms.
As of March 31, 2010, we had approximately $3,549,000 in cash and cash equivalents. Our cash balances are held in a variety of interest bearing instruments, including interest bearing demand accounts and certificates of deposit. Cash in excess of immediate requirements is invested primarily with a view to liquidity and capital preservation. We do not expect to generate product revenue from sales of our ClearPoint system products until the second half of 2010. Excluding any cash generated from sales of our ClearPoint system products, we believe the net proceeds from this offering together with our cash and cash equivalents and interest income we earn on these balances will be sufficient to meet our anticipated cash requirements through the end of 2011. If our available cash and cash equivalents and net proceeds from this offering are insufficient to satisfy our liquidity requirements, we may seek to sell additional equity or debt securities or enter into a credit facility. The sale of additional equity and debt securities may result in dilution to our stockholders. If we raise additional funds through the issuance of debt securities, these securities could have rights senior to those of our common stock and could contain covenants that would restrict our operations. We may require additional capital beyond our currently forecasted amounts. Any such required additional capital may not be available on reasonable terms, if at all. If we are unable to obtain additional financing, we may be required to reduce the scope of, delay, or eliminate some or all of, our planned research, development and commercialization activities, which could materially harm our business.
Our forecast of the period of time through which our financial resources will be adequate to support our operations, the cost to commercialize our marketed products and the costs to complete development of our product candidates are forward-looking statements and involve risks and uncertainties, and actual results could vary materially and negatively as a result of a number of factors, including the factors discussed in the “Risk Factors” section of this prospectus. We have based these estimates on assumptions that may prove to be wrong, and we could deplete our available capital resources sooner than we currently expect.
54
Because of the numerous risks and uncertainties associated with the development and commercialization of medical devices, we are unable to estimate the exact amounts of capital outlays and operating expenditures necessary to successfully commercialize our marketed products and complete the development of our product candidates. Our future capital requirements will depend on many factors, including but not limited to the following:
| • | the cost and timing of establishing sales, marketing and distribution capabilities and other corporate infrastructure; |
| • | the cost of establishing inventories; |
| • | the effect of competing technological and market developments; |
| • | the scope, rate of progress and cost of our research and development activities; |
| • | the achievement of milestone events under, and other matters related to, our agreements with Boston Scientific and Siemens; |
| • | the terms and timing of any future collaborative, licensing or other arrangements that we may establish; |
| • | the cost and timing of clinical trials; |
| • | the cost and timing of regulatory filings, clearances and approvals; and |
| • | the cost of filing, prosecuting, defending and enforcing any patent claims and other intellectual property rights. |
The following table summarizes our outstanding future contractual obligations as of December 31, 2009 and the effect those obligations are expected to have on our liquidity and cash flows in future periods:
| Payments Due by Period | |||||||||||||||
| Total | Less than 1 year |
1-3 years | 3-5 years | After 5 years | |||||||||||
| Operating Lease Obligations |
$ | 579,000 | $ | 168,000 | $ | 290,000 | $ | 121,000 | $ — | ||||||
| Long-Term Debt Obligations |
3,500,000 | — | 3,500,000 | — | — | ||||||||||
| Shared Research Obligations |
1,094,000 | 907,000 | 187,000 | — | — | ||||||||||
| Co-Development Obligations |
2,326,000 | 1,257,000 | 1,069,000 | — | — | ||||||||||
| Software License Obligations |
1,575,000 | 525,000 | 1,050,000 | — | — | ||||||||||
| Minimum Royalty Payment Obligations |
1,575,000 | 45,000 | 140,000 | 190,000 | 1,020,000 | ||||||||||
| Total |
$ | 10,649,000 | $ | 2,902,000 | $ | 6,236,000 | $ | 311,000 | $ | 1,020,000 | |||||
Our long-term commitments under operating leases shown above consist of payments relating to our facilities under leases that as of December 31, 2009 expire in 2011, 2012 and 2014. Our long-term debt obligations consist of the principal amounts owed under our convertible promissory notes issued to Boston Scientific. Shared research obligations consist of amounts payable under research agreements with certain universities. Co-development obligations consist of the payment obligations to Siemens in connection with the ClearTrace system software development. Software license obligations represent minimum purchase commitments under a master service and license agreement for the license of software code that is used in our ClearPoint system. Minimum royalty payment obligations consist of the minimum royalty payments due to a licensor.
Quantitative and Qualitative Disclosures about Market Risk
Our exposure to market risk for changes in interest rates relates to our cash equivalents on deposit in demand deposit accounts and certificates of deposit. The primary objective of our cash investment activities is to preserve principal while at the same time maximizing the income we receive from our invested cash without
55
significantly increasing risk of loss. We do not currently use derivative financial instruments. Our cash and investments policy emphasizes liquidity and preservation of principal over other portfolio considerations. We have operated solely in the United States. Accordingly, we do not have any material exposure to foreign currency rate fluctuations.
Off-Balance Sheet Arrangements
Since our inception, we have not engaged in any off-balance sheet arrangements, including the use of structured finance, special purpose entities or variable interest entities.
Recent Accounting Pronouncements
In August 2009, the FASB issued ASU No. 2009-04, Accounting for Redeemable Equity Instruments—Amendment to Section 480-10-S99, or ASU No. 2009-04. This ASU represents an update to Section 480-10-S99, Distinguishing Liabilities from Equity , per Emerging Issues Task Force Topic D-98, “Classification and Measurement of Redeemable Securities.” The adoption of ASU 2009-04 did not have a material impact on our financial statements.
In August 2009, the FASB issued ASU No. 2009-05, Fair Value Measurements and Disclosures (Topic 820)—Measuring Liabilities at Fair Value, or ASU No. 2009-05. This ASU amends Subtopic 820-10, Fair Value Measurements and Disclosures—Overall, to provide guidance on the fair value measurement of liabilities. The adoption of ASU 2009-05 did not have a material impact on our financial statements.
In October 2009, the FASB issued Accounting Standards Update No. 2009-13, or ASU 2009-13, which addresses the accounting for multiple-deliverable arrangements to enable vendors to account for products or services (deliverables) separately rather than as a combined unit. ASU 2009-13 is effective prospectively for revenue arrangements entered into or materially modified beginning in fiscal years on or after June 15, 2010. Early adoption is permitted. We are currently evaluating the impact that the adoption of this standard will have on our financial statements, if any.
In February 2010, the FASB issued authoritative guidance that amends the disclosure requirements related to subsequent events. This guidance includes the definition of a Securities and Exchange Commission filer, removes the definition of a public entity, redefines the reissuance disclosure requirements and allows companies to omit the disclosure of the date through which subsequent events have been evaluated. This guidance is effective for financial statements issued for interim and annual periods ending after February 2010. This guidance did not materially impact our results of operations or financial position, but did require changes to the disclosures in our financial statements.
In April 2010, the FASB issued Accounting Standards Update No. 2010-17, or ASU 2010-17, which provides guidance on defining a milestone and determining when it may be appropriate to apply the milestone method of revenue recognition for research or development arrangements that contain payment provisions contingent upon achieving specified events. ASU 2010-17 is effective for milestones achieved in fiscal years beginning on or after June 15, 2010. Early adoption is permitted. We are currently evaluating the impact that the adoption of this standard will have on our financial statements, if any.
56
Overview
We are a medical device company focused on the development and commercialization of technology that enables physicians to see inside the brain and heart using direct, intra-procedural MRI guidance while performing minimally invasive surgical procedures. Since our inception in 1998, we have focused on research and product development in the field of interventional MRI. From 1998 to 2002, we deployed significant resources to fund our efforts to develop the foundational capabilities for enabling MRI-guided interventions and to build an intellectual property position. In 2003, our focus shifted to identifying and building out commercial applications for the technologies we developed in prior years.
We have two core product platforms, the ClearPoint system and the ClearTrace system, both of which are designed to enable minimally invasive surgical procedures to be performed in a hospital’s existing MRI suite. We developed our ClearPoint system to perform interventional applications in the brain. We are developing the ClearTrace system to perform interventional applications in the heart. In addition, we are also focused on developing our MRI-safety technologies through the SafeLead Development Program.
We believe that our marketed products and our product candidates, subject to appropriate regulatory clearance and approval, will deliver better patient outcomes, enhance revenue potential for both physicians and hospitals, and reduce costs to the healthcare system as described in more detail below:
| • | Better Patient Outcomes. We believe that if a physician can see the surgical field, the surgical instruments and the patient’s anatomy at the same time and in the same “imaging space,” the physician can more efficiently perform a surgical intervention in the brain or heart. Our marketed products and our product candidates, subject to appropriate regulatory clearance or approval, are designed to enable physicians to identify the target site, guide the surgical instrument to the site, deliver the therapy, monitor for adverse events and complications and confirm the desired results of the procedure, all under continuous, intra-procedural, high resolution MR imaging. We believe that these capabilities will translate directly into better clinical outcomes for the patients undergoing the procedures due to improved efficiency, the potential for the reduction of adverse events and side effects, as well as the potential for faster recovery times. |
| • | Enhance Revenue Potential. Our marketed products and our product candidates are designed to enable certain procedures to be performed in the hospital’s existing MRI suite. In the case of our ClearPoint system, we believe this would make the operating room available for other procedures to be performed. The additional procedures would result in additional revenue for the hospital. In addition, we believe that use of our marketed products and our product candidates, subject to appropriate regulatory clearance and approval, will reduce the amount of time needed to perform the procedures for which they are designed. As a result, we believe that our marketed products and our product candidates will improve the overall economics of the procedures for both the performing physician and the hospital. With respect to the physician, we anticipate that the physician will receive the same fee payment for performing a procedure using our marketed products or our product candidates as for performing the procedure using conventional means. However, as we believe the procedure will take less time to perform with the use of our marketed products or our product candidates, the physician’s fee should be higher, when measured on a per hour basis. Likewise, if procedure times are shorter, the physician could perform more procedures. With respect to the hospital, we believe that shorter procedure times could lead to more efficient use of hospital resources and could increase the hospital’s capacity for more procedures. |
| • | Reduce Costs to the Healthcare System. As discussed above, we believe that use of our marketed products and our product candidates could result in better patient outcomes along with more efficient utilization of physician and hospital time and resources. If patient outcomes and procedure efficiencies are improved by use of our marketed products and our product candidates, we believe that the result will be a reduction in overall healthcare costs. If higher success rates are achieved by use of our |
57
| marketed products and our product candidates, we believe this would mitigate the need for follow-up procedures to be performed on the same patient simply to achieve the original desired clinical result. As a result, the costs associated with follow-up procedures, such as in-patient hospital stays and physician fees, would not be incurred. Similarly, if the risk for complications during the procedure is reduced, the expected costs associated with the procedure also should be reduced. |
Millions of people suffer from brain and heart diseases and disorders. While some patients can be treated with medication, some will require surgery. Current surgical interventions include both open and minimally invasive procedures. Given the option, patients, physicians and hospitals alike prefer minimally invasive procedures over open procedures. Despite the many benefits of minimally invasive procedures, they can still present significant limitations, most notably restricted vision of the patient’s anatomy, the surgical field and instruments. Because of this restricted visibility, some minimally invasive procedures in the brain and heart are lengthy, difficult on patients, and require substantial physician and hospital resources. As a result, there is a need for a new and improved platform for those procedures in the brain and heart.
Utilizing the superior imaging capabilities of MRI, our marketed products and our product candidates, subject to appropriate FDA clearance or approval, are designed to enable physicians to:
| • | Guide a surgical instrument within the patient as it is advanced towards the therapeutic target; |
| • | Deliver a planned therapy with precise visualization of a patient’s anatomy, the surgical field and instruments; |
| • | Monitor for adverse events during and immediately after the administration of the therapy; and |
| • | Confirm the desired results of a procedure. |
Our ClearPoint system is designed to allow minimally invasive procedures in the brain to be performed in the hospital’s existing MRI suite. On June 16, 2010, we received 510(k) clearance from the FDA to market our ClearPoint system in the United States for general neurological interventional procedures. We are marketing our ClearPoint system to provide guidance for the placement and operation of instruments or devices during the planning and operation of neurological procedures within the MRI environment and in conjunction with MR imaging. Our ClearPoint system is intended to be used as an integral part of procedures such as biopsies and catheter and electrode insertion, which have traditionally been performed using other methods. In the future, we may seek additional regulatory clearance or approval for use of our ClearPoint system for more specific neurological indications to allow us to market and promote our ClearPoint system for those specific uses.
We believe that one of the more valuable future applications for our ClearPoint system could be use in MRI-guided deep brain stimulation lead placement. A deep brain stimulation lead is a thin, insulated wire with exposed electrodes that is implanted in a specific area of the brain and connected to an electronic device that is implanted in the chest. Deep brain stimulation is an approved therapy for treating the symptoms of movement disorders like Parkinson’s disease and psychological disorders like treatment resistant obsessive compulsive disorder. Despite its approval for the treatment of these disorders, we believe that patient and physician adoption of deep brain stimulation therapy has been slowed significantly due to the arduous and time-consuming nature of the standard procedure by which deep brain stimulation leads are implanted in the patient’s brain. Using our ClearPoint system, a physician could see and select a neurological target, aim our targeting device and watch as the surgical instrument is advanced to the target, which we believe would significantly reduce the time and complexity of the lead implantation procedure.
The ClearTrace system is a product candidate designed to allow catheter-based minimally invasive procedures in the heart to be performed using continuous, intra-procedural MRI guidance. Some catheter-based cardiac interventions, such as stent placement, do not require detailed visualization of the cardiac tissue. However, we believe that other procedures, such as cardiac ablation to treat arrhythmias, would significantly benefit from continuous high resolution imaging of cardiac tissue and the surgical instruments. During cardiac
58
ablation, a physician attempts to restore a normal heart rhythm by destroying small areas of heart tissue to block irregular electrical impulses that cause an irregular heart beat, or arrhythmia. We expect that the ClearTrace system’s initial application will be for catheter-based cardiac ablation to treat cardiac arrhythmias, such as atrial fibrillation. Atrial fibrillation is the most common cardiac arrhythmia affecting over three million people in the United States alone. In May 2009, we entered into an exclusive co-development agreement with Siemens, the global market leader in MRI scanners, for the development and commercialization of the hardware and MRI software necessary for the ClearTrace system. Because of Siemens’ market-leading position, we believe that our exclusive relationship secures a strategic market position for the ClearTrace system. Our development activities on the ClearTrace system are ongoing. We have not made any filings seeking regulatory clearance or approval for the ClearTrace system. We anticipate that the initial market for the ClearTrace system will be the European Union.
Our other area of development is referred to as the SafeLead Development Program. Over the last ten years, we have pioneered several technologies that improve the MRI-safety profile of implantable medical leads. These leads are thin, insulated wires that are connected to implantable generators, such as a pacemaker or neurostimulator, and deliver electrical pulses or stimulation to a specific area of the body, such as the heart or the brain. During an MRI scan, these leads are susceptible to heating, which could burn and destroy adjacent tissue. Our technologies address this issue by maintaining lead temperatures well within safe levels during an MRI scan. In March 2008, we entered into an exclusive licensing and development agreement with Boston Scientific for the incorporation of our MRI-safety technologies into Boston Scientific’s implantable cardiac leads. We previously entered into a similar arrangement with Boston Scientific with respect to its products for neurological applications. Under our agreements with Boston Scientific, we received licensing fees of $13,000,000 in 2008 and we are entitled to receive up to approximately $21,600,000 in future milestone-based payments, subject to our achievement of the milestones stipulated in the agreements and the issuance of certain patents licensed to Boston Scientific. Boston Scientific has also agreed to pay us royalties on net sales of products that incorporate our licensed intellectual property. We believe that our MRI-safety technologies, when integrated into Boston Scientific’s implantable leads, could represent a meaningful market differentiator over existing implantable lead designs.
Our ClearPoint system and the ClearTrace system are integrated systems of reusable components, disposable components and intuitive, menu-driven software. Our business model for both the ClearPoint and ClearTrace systems is focused on producing high margin revenue from recurring sales of the disposable components. We intend to make our reusable components available to hospitals at lower margins. In addition, the reusable components can be installed at minimal cost to the hospital, without disrupting the hospital’s routine schedule for use of its MRI scanner. We do not expect that the cost of the reusable components of our ClearPoint system and the ClearTrace system or the cost of installation of such reusable components will negatively impact the adoption rate of our systems among hospitals.
We have a significant intellectual property portfolio in the field of MRI-guided interventions. In addition, we have meaningful collaborations with major industry participants and renowned academic institutions. Our technologies have been the subject of numerous peer-reviewed articles in medical and scientific journals. As a result of our intellectual property and collaborative relationships, we believe that we are well positioned to remain on the forefront of the emerging market of MRI-guided minimally invasive surgical procedures.
Recent Developments
No material development has occurred since the conclusion of our quarterly period ended March 31, 2010 with the exception of our receipt of FDA clearance to market our ClearPoint system, which is discussed elsewhere in this prospectus.
Industry Background
Development of Minimally Invasive Surgical Procedures
Over the past few decades, one of the most significant medical trends has been the development of minimally invasive surgical methods and techniques. As its name implies, a minimally invasive procedure is a
59
less invasive approach than open surgery. Minimally invasive procedures typically have involved use of laparoscopic devices, catheter-based devices or remote-control manipulation of instruments once inside the body.
Compared to open surgical techniques, minimally invasive techniques offer potentially superior benefits for patients, physicians and hospitals:
| • | For the patient, these techniques result in reduced procedure-related pain, minimal scarring and reduced pain at the incision site, shorter post-operative hospital stays and faster recovery times; |
| • | For the physician, these techniques reduce procedure-related complications and have the potential to reduce risks associated with more invasive procedures; and |
| • | For the hospital, these procedures result in reduced hospital stays with faster recovery times, lower rates of complications, and reduced costs. |
Procedures commonly performed using minimally invasive techniques include knee surgery, gastric surgery, cardiovascular balloon angioplasty, stent placement and tumor biopsy. In the United States alone, approximately 4.9 million minimally invasive surgical procedures are performed annually.
One of the ongoing challenges of minimally invasive procedures is the physician’s ability to “see” what he or she is doing inside a patient’s body. Technological advances in imaging modalities that permit a physician to see inside a human body have enabled the development and growth of minimally invasive surgical procedures. One such imaging modality is endoscopy, which involves examining the inside of a person’s body using a long, thin, flexible tube with a light and a video camera, which displays images of the inside of the patient’s body on a screen. The development of endoscopic visualization techniques reinvented the manner in which knee surgery was performed. Another imaging modality is fluoroscopy, which uses a continuous X-ray beam to create a sequence of images that are projected onto a television-like monitor. Fluoroscopic imaging enabled revolutionary improvements in the treatment of cardiovascular disease by guiding balloon angioplasty procedures and the placement of cardiac stents. The development of endoscopic and fluoroscopic techniques have dramatically increased the number of procedures performed when compared to the number of open procedures previously performed. While endoscopic and fluoroscopic imaging techniques are optimal for some minimally invasive procedures, we believe that many procedures in the brain and heart would benefit from a different imaging platform.
Magnetic Resonance Imaging
MRI is a widely practiced imaging technique that uses spatially varying magnetic fields to produce images of the human anatomy. Hydrogen nuclei, present in molecules throughout the body, are slightly magnetic. When placed in large external magnetic fields, they can be induced to emit or resonate radio frequency signals. These radio frequency signals are used to construct images of human anatomy, including high resolution images of soft tissue.
MRI has important and advantageous properties that differentiate it from other imaging methods. MRI scans can provide images of any part of the body, in any plane of view, and offer more detailed information than other modalities, including fluoroscopy and computed tomography. Some of the unique advantages of MRI include:
| • | no harmful ionizing radiation exposure for either the patient or the physician; |
| • | soft tissue imaging that enables superior tissue visualization and enhanced differentiation between healthy and diseased tissues; |
| • | unlimited orientation and positioning of the imaging plane; |
| • | ability to directly acquire volumetric (three dimensional) data sets; and |
| • | ability to evaluate both the structure and certain functions of internal organs. |
MRI scanners are available in a number of different configurations and field strengths, which refers to the strength of the magnet used to create the magnetic field. Magnetic field strength is measured in Tesla, or T. The
60
most common field strength for MRI scanners is 1.5T. Most MRI scans are performed using 1.5T MRI scanners. Higher field strength scanners such as 3T MRI scanners have been introduced in clinical practice and are in the early stages of commercial market adoption. These 3T MRI scanners provide faster scanner speeds and even higher resolution images than 1.5T MRI scanners.
The SurgiVision Solution
The last 20 years have witnessed significant advances in minimally invasive surgical techniques. However, we believe that some minimally invasive procedures within the brain and heart have been slow to develop and gain wide acceptance largely because of the inherent limitations of traditional imaging methods such as endoscopy or fluoroscopy. Neither of these imaging methods provides the physician with sufficient visualization of the brain or heart tissue to perform the next generation of minimally invasive neurological and cardiac procedures. Utilizing the power of MRI, our marketed products and our product candidates provide that capability. Our marketed products and our product candidates, subject to appropriate regulatory clearance or approval, are designed to enable physicians to:
| • | Guide the surgical instrument within the patient as it is advanced towards the therapeutic target. For example, a physician will be able to watch a probe as it moves through the brain towards its target point or visualize and steer a catheter into a chamber of the heart. |
| • | Deliver the planned therapy with continuous high resolution visualization of a patient’s anatomy, the surgical field and instruments. For example, a physician will be able to visualize ablation lesions in the heart as the physician creates them. |
| • | Monitor for adverse events during and immediately after the administration of the therapy. For example, if a blood vessel in the brain is ruptured, hemorrhage will be visible within seconds and remedial action can be undertaken immediately. |
| • | Confirm the desired results of a procedure. For example, a physician will be able to confirm, with specificity, correct anatomical placement of a device or delivery of a therapy in the brain or heart. |
We believe the combination of MRI’s continuous, high resolution imaging capabilities with minimally invasive surgical techniques will create an innovative platform for performing the next generation of procedures in the brain and heart.
Our Marketed Products
ClearPoint Neuro Intervention System
General
Our ClearPoint system is designed to allow minimally invasive procedures in the brain to be performed in the hospital’s existing MRI suite. Our research efforts for our ClearPoint system began in 2003. On June 16, 2010, we received 510(k) clearance from the FDA to market our ClearPoint system in the United States for general neurological interventional procedures. We are marketing our ClearPoint system to provide guidance for the placement and operation of instruments or devices during the planning and operation of neurological procedures within the MRI environment and in conjunction with MR imaging. Our ClearPoint system is intended for use with 1.5T MRI scanners, and it is intended to be used as an integral part of procedures such as biopsies and catheter and electrode insertions, which have traditionally been performed using other methods. In the future, we may seek additional regulatory clearance or approval for use of our ClearPoint system for more specific neurological indications to allow us to market and promote our ClearPoint system for those specific uses. Such additional regulatory clearances or approvals may require us to perform clinical studies.
We believe that one of the more valuable future applications for our ClearPoint system could be MRI-guided deep brain stimulation lead placement. Deep brain stimulation is an approved therapy for treating the symptoms of movement disorders such as Parkinson’s disease and psychological disorders like treatment
61
resistant obsessive compulsive disorder. Despite its approval for the treatment of such disorders, we believe that patient and physician adoption of deep brain stimulation therapy has been slowed significantly due to the arduous and time-consuming nature of the standard procedure by which deep brain stimulation leads are implanted in the patient’s brain. Using our ClearPoint system, a physician could see and select a neurological target, aim our targeting device and watch as the surgical instrument is advanced to the target, which we believe would significantly reduce the time and complexity of the interventional procedure.
Another future ClearPoint system application for which we may seek FDA clearance or approval is the delivery of specific drugs and biologic agents to precision targets in the brain to treat a variety of neurological diseases and conditions, including brain tumors. We believe that many therapies are currently not available because the drugs and biologic agents cannot be delivered effectively to their neurological targets. Delivery challenges include penetration of the blood-brain barrier, which is a protective barrier between brain tissue and circulating blood preventing some substances from entering the brain, and the risk of serious side effects which can occur if the drugs or biologics are unintentionally delivered to the tissue that surrounds the intended target site. We believe that our ClearPoint system could address these significant issues.
Components
Our ClearPoint system is an integrated system of reusable components, disposable components and intuitive, menu-driven software. Pictures of some of our ClearPoint system components are included on the inside front cover of this prospectus.
Reusable Components. Our reusable components consist primarily of an imaging head coil, head fixation frame, computer workstation and in-room monitor. The architecture of our imaging head coil allows for surgical access to the patient while maintaining high quality imaging capability. The head fixation frame is integrated with the head coil and is designed to optimize the placement of the head coil in proximity to the patient’s head. Our ClearPoint system software is installed on a computer workstation networked with an MRI scanner, for which we use a commercially available laptop computer. The in-room monitor allows the physician to view the display of our ClearPoint system workstation from the scanner room while performing the procedure.
Disposable Components. Our hardware components consist primarily of our SmartFrame device, a hand controller and a surgical kit. Our SmartFrame device is an adjustable trajectory frame that attaches to the patient’s skull and that holds the targeting cannula. The hand controller attaches to our SmartFrame device, and it is used by the physician to adjust the roll, pitch and X and Y orientation of the targeting cannula. The surgical kit includes all other accessory components necessary for the MRI-guided neurological procedure, such as our SmartGrid patch, which is an MRI-visible marking grid, and customized surgical draping.
Software. Our ClearPoint system software guides the physician in surgical planning, device alignment, navigation to the target and procedure monitoring. The software receives standard images from the MRI scanner via a network connection to the scanner. The software leads the physician through a series of predefined steps, including MR image acquisition, establishment of image orientation landmarks, target identification and selection, trajectory planning, entry point planning and marking, targeting cannula orientation and refinement, and confirmation that the desired anatomical target(s) have been reached. The software uses image segmentation algorithms to help locate and identify our SmartFrame device and its targeting cannula, the probe and the anatomical structures of the brain. The software also performs geometric computations to provide the physician with information regarding the positioning of instruments inserted into the patient’s brain relative to the target anatomical structures. At the completion of the procedure, the software generates an automated report that includes the key metrics from the procedure. Our ClearPoint system software will be included as part of the initial installation of our ClearPoint system pursuant to an end-user license agreement.
62
Current Neurological Interventions
Performing minimally invasive interventions in the brain presents special challenges, including a need to reach small therapeutic targets often located deep within the brain. To reach these targets, the physician must act with precision to avoid damaging adjacent areas that are responsible for important neurological functions, such as memory or speech, or penetrating blood vessels which can lead to a life-threatening hemorrhage. To overcome these obstacles, the medical community has developed complicated surgical techniques, commonly referred to as stereotaxy, under which a physician merges pre-operative images and data with specialized surgical instruments to help guide the surgical intervention. Despite years of development and clinical experience, conventional stereotactic procedures remain complicated and time-consuming for many neurological interventions and can be extremely difficult on the patient.
In spite of their shortcomings, current stereotaxy-based approaches are commonly used to perform neurological interventions. These procedures include pre-operative biopsy and the insertion of catheters or electrodes in the brain. In 2007, industry analysts estimated that over 130,000 minimally invasive neurological interventions would be performed in the United States in 2008, including approximately 17,000 biopsies, 75,000 catheter insertions, and 8,000 electrode insertions. We believe our ClearPoint system is an innovative new approach to perform a subset of these neurological procedures.
Our ClearPoint System Solution
The design of our ClearPoint system significantly simplifies how neurological interventions are performed. Our solution, unlike some conventional approaches, begins with the patient in an MRI suite under general anesthesia and without interruption to the patient’s prescription drug regimen. Once placed in the MRI scanner, the patient’s head is immobilized in our imaging head coil and integrated head fixation frame with the patient’s head accessible to the surgeon. The physician then places our MRI-visible SmartGrid patch onto the patient’s head where the physician expects to enter the skull.
The patient is then moved to the center of the scanner and images are taken of the patient’s brain that include the target area and our SmartGrid patch. Once the imaging is complete, the images are transferred to our ClearPoint system workstation so that the physician can determine the specific target site within the brain and the optimal trajectory path for the placement of the interventional device. With the trajectory path established, our ClearPoint system software will identify the specific location on our SmartGrid patch that corresponds with where the planned trajectory intersects the skull. The physician will then mark the skull using our custom marking tool. At the site of the mark, the physician will create a small 14 millimeter hole, which is called a burr hole, in the patient’s skull.
The SmartFrame device is centered and attached over the burr hole. The target and planned trajectory is reconfirmed by the physician using our ClearPoint system workstation. Using the hand controller, the physician positions the MRI-visible SmartFrame device to align the instrument with the planned trajectory. During this process, the software estimates a number of turns and direction of turn on each of the hand controller’s color coded thumbwheels to align the instrument to the planned trajectory.
Once our SmartFrame device has been aligned to the proper trajectory, the depth dimension is calculated by the software. Immediately before insertion and partway through insertion, scans are taken to ensure that the stylet is correctly tracking along the planned trajectory. The surgeon continues advancing the stylet towards the target site until it “snaps” into place on the SmartFrame device indicating that the stylet has reached the proper depth. At this time, images are taken at the target site to insure the peel away sheath and stylet are in the proper location relative to the desired target. Once proper location is confirmed, the stylet is removed, leaving behind a channel to the target site created by the peel away sheath. Now the interventional device can be inserted and the peel away sheath is removed.
63
Potential Future Applications for our ClearPoint System
Deep Brain Stimulation
Deep brain stimulation is a therapy that uses mild electrical pulses from an implanted device to stimulate the brain. A deep brain stimulation system looks and operates much like a cardiac pacemaker, except that instead of sending pulses to the heart, it delivers electrical stimulation to a precisely targeted area in the brain. Several types of medications are available as the first line of treatment for these conditions. However, over time, these medications often become less effective at controlling symptoms and may begin to cause side effects. For those patients who fail to respond to, or have developed side effects from, standard drug therapies, deep brain stimulation can be an appropriate therapy.
To date, more than 60,000 people worldwide have undergone a deep brain stimulation procedure. The FDA has approved the use of deep brain stimulation for the treatment of Parkinson’s disease and essential tremor. The FDA has also approved the use of deep brain stimulation for the treatment of dystonia and obsessive compulsive disorder pursuant to humanitarian device exemptions. We believe that the market for deep brain stimulation therapy is sizable because of the number of people suffering from these diseases or disorders for whom deep brain stimulation may be an appropriate treatment. FDA approval is currently being sought for the use of deep brain stimulation to treat epilepsy. Deep brain stimulation is also being investigated as a therapy for treatment-resistant major depression. We believe that the market for deep brain stimulation is growing because FDA approval is being sought and investigations are being conducted for these additional uses of deep brain stimulation therapy. The diseases and disorders, patient populations, potential deep brain stimulation candidates and status of FDA approval are described in the following table:
United States Deep Brain Stimulation Market
| Indication |
Patient Population |
Potential DBS Candidates(1) |
FDA Approval |
||||
| Parkinson’s Disease |
1,500,000 | 150,000 | Approved | ||||
| Essential Tremor |
4,000,000 | 75,000 | Approved | ||||
| Dystonia |
250,000 | 25,000 | Approved | (2) | |||
| Obsessive Compulsive Disorder |
3,300,000 | 330,000 | Approved | (2) | |||
| Epilepsy |
2,300,000 | 460,000 | Pending | ||||
| Treatment-Resistant Major Depression |
6,000,000 | 1,200,000 | Unknown | (3) | |||
| Subtotal |
17,350,000 | 2,240,000 |
| (1) | The number of potential deep brain stimulation candidates are based on publicly available industry research reports and third-party corporate presentations. |
| (2) | Pursuant to a Humanitarian Device Exemption—Efficacy has not been established. |
| (3) | Although this indication is being actively investigated for deep brain stimulation therapy, no submissions have been filed with the FDA seeking approval and there can be no assurance that approval will ever be sought or received. |
Conventional Deep Brain Stimulation Lead Placement Procedure
Despite the large potential market for deep brain stimulation and many years of research experience with deep brain stimulation technology, we believe that the conventional deep brain stimulation lead placement procedure has led to an under-developed market. The current approach for implantation of deep brain stimulation leads is a complex and lengthy procedure that is performed in an operating room. We believe that many patients identified by their physicians as candidates for deep brain stimulation therapy elect not to proceed with the treatment because of the arduous aspects of the procedure, namely:
| • | The patient is awake for his or her own brain surgery; |
| • | The patient’s head is affixed to a large, metal frame by skull pins; |
64
| • | A Parkinson’s patient must stop taking medication prior to the procedure, which can result in uncontrolled body tremors during the procedure; and |
| • | The procedure can last more than six hours. |
The standard deep brain stimulation lead implantation approach is based on a technique called frame-based stereotaxy. In this method, a large, metal stereotactic frame is fixed to the patient’s skull, using skull pins, to provide a fixed and common coordinate system. After the frame is attached to the patient’s skull, the patient is then imaged pre-operatively in order to obtain images showing both the stereotactic frame axes and the anatomical structures of the patient’s brain. These pre-operative images are then loaded into a surgical planning workstation. Surgical planning software is used to identify the neurological target for the deep brain stimulation therapy, as well as to define a trajectory path for the deep brain stimulation lead from the skull, through the brain tissue, and to the target. The planned trajectory and target location is then calculated in relation to the frame axes and then used to guide the surgery.
Successful deep brain stimulation therapy requires a high degree of accuracy in the placement of deep brain stimulation leads within specific deep brain structures. Because frame-based stereotaxy relies on pre-operative images, and not intra-procedural images, errors in the alignment of the pre-operative images with the patient’s brain anatomy can, and often do, occur as a consequence of brain shift, variation in patient hydration, registration errors or misalignment of the frame. As a result, the physician often must undertake additional steps to further refine the process of locating the patient’s neurological targets. These steps include physiological “mapping” of the brain and require an additional procedural step called microelectrode recording, which is a tedious and time-consuming process during which small probes containing microelectrodes are inserted into the deep brain structures usually multiple times. As these microelectrode recording probes are passed through brain tissue, they pick up electrical activity. The microelectrode recording system then converts the electrical activity into audible tones. In hearing these various audible tones, a trained neurologist or neurophysiologist can distinguish different regions of the brain. Based on these tones, locations are mapped against the pre-operative images and used to refine and adjust the neurological target as depicted on those pre-operative images. New coordinates are then calculated and a new trajectory is planned. To further confirm locations in the brain, various physiologic responses are induced or monitored with the microelectrodes. These physiological mapping steps require the patient to be awake and off medications.
A ClearPoint System Procedure
We believe that deep brain stimulation therapy would benefit from simplified lead implantation methodologies and that our ClearPoint system represents a dramatic improvement over the current approach. We believe that a deep brain stimulation lead implantation procedure utilizing our ClearPoint system would have the following attributes:
| • | the patient will not need to be awake and can be under general anesthesia and remain on his or her medication; |
| • | the procedure will be performed in a standard 1.5T MRI scanner; |
| • | intra-procedural MRI-guidance will be used to: |
| • | see and select the target site; |
| • | plan the path to the target site; |
| • | guide an instrument to the target site; |
| • | monitor for any adverse events or complications that might occur during the procedure; and |
| • | confirm that the target site has been reached; and |
| • | the procedure is designed to be completed in approximately two to three hours. |
We believe that the process of a deep brain stimulation lead implantation procedure utilizing our ClearPoint system would be substantially similar to the process described above in “—Our ClearPoint System Solution” on page 63.
65
Precision Delivery of Drugs and Biologics
Another potential future ClearPoint system application for which we may seek FDA clearance or approval is the delivery of specific drugs and biologic agents to precision targets in the brain. Recently, drug companies and researchers have identified various compounds that may treat a number of neurological diseases, including movement and psychiatric disorders and brain tumors. Based on peer reviewed articles in medical and scientific journals, we believe that some of these therapies are currently not available because the drugs and biologic agents cannot be delivered effectively to their neurological targets. Delivery challenges include penetration of the blood-brain barrier and the risk of serious side effects which can occur if the drugs and biologics are unintentionally delivered to the tissue that surrounds the intended target site. We believe our ClearPoint system could address these significant issues.
We are presently conducting animal studies in close collaboration with renowned researchers in the field. These preliminary studies are demonstrating our ClearPoint system’s capability to allow the physician to identify a precise neurological target area, guide an injection catheter into the target area, and watch the dispersion of the material within the target area as it is injected. We believe these capabilities for precision delivery are unique and remove a major barrier that has been preventing promising therapies from reaching the market.
Regulatory Status
On June 16, 2010, we received 510(k) clearance from the FDA to market our ClearPoint system in the United States for use in general neurological interventions, which is the same indication for use that applies to other devices that have traditionally been used in the performance of stereotactic neurological procedures. We are marketing our ClearPoint system to provide guidance for the placement and operation of instruments or devices during the planning and operation of neurological procedures within the MRI environment and in conjunction with MR imaging. Our ClearPoint system is intended for use with 1.5T MRI scanners, and it is intended to be used as an integral part of procedures such as biopsies and catheter and electrode insertion, which have traditionally been performed using other methods. In the future, we may seek additional regulatory clearance or approval for use of our ClearPoint system for more specific neurological indications, such as deep brain stimulation lead placement or precision delivery of drugs or biologics, to allow us to market and promote our ClearPoint system for those specific uses. Any such additional regulatory clearances or approvals may require us to perform clinical studies.
Unless and until we receive regulatory clearance or approval for use of our ClearPoint system for specific indications, uses in procedures other than general neurological interventions, such as biopsies and catheter and electrode insertion, may be considered off-label uses of our ClearPoint system, in which case we would be prohibited from promoting our system, or training physicians, for those specific uses. However, in their practice of medicine, physicians may lawfully choose to use medical devices for off-label uses. Therefore, a physician may use our ClearPoint system for uses not covered by the cleared labeling. We expect that physicians will use our ClearPoint system for a variety of specific neurological procedures, including deep brain stimulation lead placement.
To market our ClearPoint system in the European Union, we must be entitled to affix a CE mark, an international symbol of adherence to quality assurance standards and compliance with applicable European Union medical device directives. We intend to apply for CE marking approval for sale of our ClearPoint system during 2010. We have engaged KEMA as the Notified Body for our CE marking approval process. A Notified Body is a private commercial entity that is designated by the national government of a European Union member state as being competent to make independent judgments about whether a device complies with applicable regulatory requirements. The exact regulatory pathway for CE marking approval will be the subject of discussions that we have with KEMA. At this time, we are unable to accurately predict when, if ever, CE marking will be obtained, whether clinical trials will be required as part of the CE marking approval process or the regulatory requirements to which we would be subject after approval.
66
Our Product Candidates
The following table summarizes key information about our product candidates in development:
| Product Candidates |
Regulatory Status |
Target Market |
Development Partner | |||
| ClearTrace Cardiac Intervention System | Development Stage | Initial target market is catheter-based cardiac ablation to treat cardiac arrhythmias, such as atrial fibrillation. Subsequent target markets may include precision delivery of drugs and biologics. | Siemens | |||
| SafeLead Development Program(1) | Development Stage(2) | Target market is implantable leads for cardiac and neurological applications. | Boston Scientific | |||
| (1) | The SafeLead Development Program is a collaborative effort with Boston Scientific to incorporate our MRI-safety technologies into Boston Scientific’s implantable lead designs. |
| (2) | Boston Scientific is responsible for any regulatory filings with respect to its implantable leads. |
The ClearTrace Cardiac Intervention System
General
The ClearTrace system is a product candidate designed to allow catheter-based minimally invasive procedures in the heart to be performed using continuous, intra-procedural MRI guidance. Catheter-based cardiac interventions performed in a fluoroscopy suite, generally referred to as a Cath Lab, have been the standard of care for the treatment of many cardiac disorders, such as cardiovascular disease. Some of these procedures, such as stent placement, are well suited for fluoroscopic imaging because they do not require continuous, detailed visualization of the cardiac tissue. However, other procedures are not well suited for fluoroscopy because of the clinical need for continuous, high resolution imaging of the cardiac anatomy along with the interventional instruments. One example of such a procedure is cardiac ablation to treat cardiac arrhythmias, such as atrial fibrillation, which is typically performed in a specialized suite referred to as an EP Lab. Another example is the precision delivery of biologics, including stem cells and gene therapies, directly into the wall of the heart, which represents a promising therapy being researched for the treatment of heart failure.
The ClearTrace system will be similar to the conventional Cath Lab or EP Lab, but with two critical distinctions. First, unlike the Cath Lab or EP Lab, the ClearTrace system will provide a continuous, four dimensional imaging environment (the fourth dimension being time), which will include detailed visualization of cardiac tissue, along with the cardiac catheters used to deliver the therapy. We believe that this capability is required for the next generation of interventional cardiac therapies. Second, the ClearTrace system will eliminate all radiation exposure for both the patient and physician from the X-ray utilized in current procedures. Under current catheter-based treatments utilizing fluoroscopy, radiation exposure can exceed 45 minutes. We believe that the attributes of the ClearTrace system position it to be the therapy of choice for cardiac ablation procedures to treat cardiac arrhythmias, including atrial fibrillation, and the ideal platform for delivering future biologic therapies to treat heart failure and other similar cardiac disorders. The ClearTrace system is designed for procedures that initially will be performed using a 3T MRI scanner.
We began preliminary research for an MRI-guided cardiac ablation procedure shortly following our inception in 1998. As a culmination of our research efforts, in May 2009, we entered into an exclusive co-development agreement with Siemens, the global market leader in MRI scanners, for the development and commercialization of the hardware and MRI software necessary for the ClearTrace system. Under the terms of this agreement, we are working together with Siemens on the development of the ClearTrace software and the integration of system components. Once product development is completed, we will work together with Siemens on the commercial launch and field support of the ClearTrace system. Because of Siemens’ market-leading position, we believe that our exclusive relationship secures a strategic market position for the ClearTrace system.
67
Components
The ClearTrace system is an integrated system of reusable components, disposable components and intuitive, menu-driven software.
Reusable Components. Our primary reusable component is our ClearConnect system, which is an MRI-compatible hardware and cable management system to safely enable MRI-guided cardiac ablation procedures in a Siemens 3T MRI scanner.
Disposable Components. Our disposable components consist primarily of a septal puncture kit, mapping catheter, a coronary sinus catheter and ablation catheter. Our septal puncture kit consists of a septal puncture needle, a dialator and sheath and will be used to perform an MRI-guided puncture of the septum of the heart to allow movement between the right atrium and left atrium. Our mapping catheter will be used for MRI-guided collection of intracardiac electrocardiogram signals and will include analog/digital filtering to enable electrocardiogram collection during scanning. Our coronary sinus catheter will be used to collect additional electrocardiogram signals and to provide cardiac pacing and defibrillation, as needed during the procedure. Our ablation catheter will be used to perform MRI-guided delivery of ablative energy to create cardiac lesions. All catheters and components will be MRI-compatible and tightly integrated with the MRI scanner.
Software. The ClearTrace system includes intuitive, menu-driven software to assist the physician in: surgical planning; creating three dimensional volumes of cardiac chambers; navigating our ClearTrace catheters within the cardiac chambers; visualizing lesions as they are formed; tracking of prior lesion locations; evaluating ablated cardiac tissue; and monitoring for possible adverse events. Under our co-development agreement, Siemens is responsible for developing the ClearTrace system software to our specifications. The ClearTrace system software will be integrated with our disposable components.
Current Atrial Fibrillation Treatments
Cardiac arrhythmia is an abnormal beating of the heart that can result in insufficient blood flow, which may cause dizziness, inadequate function of important organs in the body, stroke and even death. Atrial fibrillation affects over three million people in the United States alone, making it the most common form of cardiac arrhythmia. Atrial fibrillation is characterized by the irregular fluttering and/or very rapid beating of the atria resulting from the malfunctioning of the electrical conduction system in the walls of the atria. Atrial fibrillation is a leading cause of stroke among persons 65 years or older and it is associated with increased risk of morbidity and mortality as well as a reduced quality of life.
Most atrial fibrillation treatments are palliative and do not cure atrial fibrillation. The most common are anti-arrhythmic and anticoagulant drugs. However, anti-arrhythmic drug therapy often becomes less effective over time, with approximately half of the patients developing resistance to the drugs. In addition, anti-arrhythmic drugs have potentially severe side effects, including pulmonary fibrosis, impaired liver function, thyroid problems and the development of worse and even life-threatening ventricular arrhythmias.
One highly effective, curative therapy for atrial fibrillation used today is an open-heart operation, commonly known as the surgical “Cox-Maze” procedure, which has reported success rates as high as 96%. During this open heart procedure, the physician makes a series of cuts in a specific “maze-like” formation along the inside walls of the left atrium with a scalpel, and then sutures these cuts back together. The scars create an uninterrupted conduction block containing the chaotic electrical impulses that cause atrial fibrillation, thereby returning the heart to a normal rhythm. The open heart Cox-Maze procedure is usually done in tandem with another open heart procedure, such as a valve replacement or coronary artery bypass, because this operation is traumatic to the patient, very expensive, and typically associated with long hospital stays and a three to six month recovery time.
Because of the effectiveness of the Cox-Maze method, the medical community has been working for years to develop a less invasive approach that generates comparable clinical outcomes. The current minimally invasive
68
approach is performed in the EP Lab with the physician relying upon flouroscopic imaging to guide a catheter through a blood vessel into the right atrium, puncturing the septum and advancing the catheter into the left atrium of the heart. The physician then delivers energy through the catheter to create lesions and destroy the target tissue. During the procedure, the physician is assisted in guiding and positioning the catheter primarily by fluoroscopic imaging. However, fluoroscopic imaging has significant limitations, namely it does not permit the physician to see the cardiac anatomy and tissue, the location of the catheter in relation to the cardiac tissue, or the intra-procedural creation of the lesions necessary to create the conduction block. Furthermore, the use of fluoroscopy exposes both patient and physician to dangerous radiation for an extended period of time.
The open Cox-Maze procedure has been considered the gold standard for surgical treatment of atrial fibrillation with reported success rates as high as 96%. However, because the Cox-Maze procedure is highly invasive, it is infrequently used as a stand alone therapy to treat atrial fibrillation. The current catheter-based approach is promising due to its less invasive nature, but the approach has been hampered by disappointing success rates, some as low as 50% to 75%. We believe that the success rate of the current catheter-based approach is dramatically lower because the physician cannot see the cardiac tissue.
The ClearTrace System Solution
The ClearTrace system represents a new paradigm in performing cardiac interventions by using MRI to allow the physician to see the cardiac tissue, as if performing an open heart Cox-Maze procedure, but with a minimally invasive approach. The ClearTrace system offers a novel, comprehensive solution for the planning, delivering and intra-procedural assessment of catheter-based cardiac interventions. The following discussion outlines the key steps in performing a ClearTrace system procedure to treat atrial fibrillation.
At the start of a ClearTrace procedure, a MRI scan is performed of the patient’s heart and surrounding vasculature. Using the images from the scan, the ClearTrace system software generates a three dimensional volumetric model of the patient’s cardiac chambers that the physician will use as a guide while performing the procedure. Additional MRI images and patient data can be mapped onto the surface of the three dimensional model as needed by the physician. Referencing the three dimensional model and surface mapped image data and using real time MRI scans of the patient’s heart, the physician plans the cardiac ablation procedure.
The ClearTrace coronary sinus catheter is then advanced through a blood vessel under MRI guidance and placed in the coronary sinus to collect electrocardiogram signals and to provide cardiac pacing and defibrillation, as may be needed during the procedure. The remaining ClearTrace catheters are then advanced through a blood vessel under MRI guidance into the right atrium of the heart. Using the ClearTrace system plan, the physician will advance the catheters through the targeted site on the septum and into the left atrium. Referencing the ablation plan, and with continuous intra-procedural visualization of the catheters and patient anatomy, the physician will advance the catheters to the site of the first planned ablation. With the ClearTrace ablation catheter in the correct location, the physician will begin applying energy to the tip of the catheter to create a lesion.
During ablation, the ClearTrace system will present intra-procedural MR images that will allow the physician to see the changes in the tissue caused by the ablative energy, giving the physician the visualization capabilities similar to what he or she has in the open heart Cox-Maze procedure. The physician will then repeat the process of creating and visualizing lesions within the left atrium until the ablation plan has been completed. The physician will complete the procedure by taking a final scan to confirm the proper placement of all lesions.
By allowing the physician to see the lesions during the procedure, we believe the physician can make better decisions about where to ablate, what amount of energy to apply and how long to apply the energy. We believe this improved decision making capability will result in improved outcomes and reduced adverse events. In addition to the ability to visualize the changes in the cardiac tissue, the physician will also be able to use a loop catheter to measure electrical signals from the inside surface of the left atrium to further guide and confirm the effectiveness of the ablation process.
69
Other Potential Applications
We believe the ClearTrace system’s unique ability to provide continuous, high resolution imaging of the cardiac anatomy, including the walls of the heart, during an interventional procedure will be valuable in treating other cardiac disorders. For example, we believe the ClearTrace system could serve as an ideal platform for delivering drugs and biologics directly into the heart wall. The medical community is developing novel compounds that have the potential to address significant cardiac disorders, such as heart failure. However, some of these compounds must be injected directly into the heart wall with precision placement at the boundary of healthy and diseased tissue. Using the ClearTrace system, a physician will be able to navigate within the heart to the boundary between healthy and diseased tissue, place the catheter tip on the boundary, inject the compound and watch the dispersion of the compound into the heart wall.
Regulatory Status
We are still in the early stages of the development of the ClearTrace system, and we have made no filings seeking appropriate regulatory approval or clearance for the ClearTrace system in the United States or in any foreign jurisdiction. In the United States, we believe that most components of the ClearTrace system will be Class II medical devices and will fall under the FDA’s 510(k) regulatory process. However, the ablation catheter component will be a Class III medical device and will require FDA approval of a PMA. We anticipate that the initial market for the ClearTrace system will be the European Union, and we plan to seek CE marking approval for the ClearTrace system. Whether as part of the PMA process in the United States or the CE marking approval process in the European Union, we expect to conduct a clinical trial regarding the safety and effectiveness of our ablation catheter, and we expect to commence enrollment in such a clinical trial in the second half of 2011.
SafeLead Development Program
Our other area of development is referred to as the SafeLead Development Program. Over the last ten years, we have pioneered several technologies that improve the MRI-safety profile of implantable medical leads. These leads are thin, insulated wires that are connected to implantable generators, such as a pacemaker or neurostimulator, and deliver electrical pulses or stimulation to a specific area of the body, such as the heart or the brain. The current market for active implantable cardiac and neurological devices exceeds $11 billion in annual revenues with more than 500,000 devices implanted per year.
It is estimated that between 50% and 75% of patients with an implantable device are expected to need an MRI scan during the lifetime of their devices. However, implantable medical leads are susceptible to heating in the MRI environment. An MRI scanner transmits radio frequency energy during the scanning process. Because the implantable lead contains metallic wire, which acts like an antenna, some of the radio frequency energy transmitted by the MRI scanner is absorbed by the lead. This could cause the lead to heat. The extent to which an implantable lead may heat can depend on many factors, such as the lead itself, the position of the patient in the MRI scanner, the clinical scanning sequence used and the location and trajectory of the lead in the patient. Scientific studies have shown that implantable leads may heat during an MRI scan to temperatures that can burn or destroy tissue. If that happens in the heart or brain, the patient could suffer a stroke, paralysis or even death. As a result, people with active implantable devices are prohibited from undergoing an MRI scan.
Our technologies address this issue by maintaining lead temperatures well within safe levels during an MRI scan. Current safety standards for active implantable medical devices require that MRI-related heating may not exceed one degree Celsius in the brain and two degrees Celsius in the heart. Our testing has shown that our technologies limit lead heating to less than one degree Celsius. Therefore, we believe our MRI-safety technologies will permit a patient with an implantable medical device to undergo an MRI scan. Manufacturers’ studies have shown that cardiologists identify “MRI compatibility” as one of the main features that would drive a change in brand preference.
70
While we have been developing our MRI-safety technologies underlying the SafeLead Development Program for the last ten years, the SafeLead Development Program commenced in December 2005 when we signed our first agreements with Boston Scientific for the incorporation of our MRI-safety technologies into Boston Scientific’s implantable leads for neurological applications. In March 2008, we entered into similar agreements with Boston Scientific for the incorporation of our MRI-safety technologies into Boston Scientific’s implantable cardiac leads. In connection with the cardiac agreements, we received licensing fees of $13,000,000 in 2008. In addition, we are entitled to receive up to $21,600,000 in future milestone-payments under both the cardiac and neuro agreements, subject to our achievement of the milestones stipulated in the agreements and the issuance of certain patents licensed to Boston Scientific. Boston Scientific has also agreed to pay us royalties on net sales of products that are covered by a licensed patent. We believe our safety technologies, if integrated into Boston Scientific’s implantable leads, could represent a meaningful market differentiator over existing implantable lead designs.
The SafeLead Development Program is still in its developmental stages and no regulatory filings have been made with the FDA or any foreign authority. Boston Scientific is responsible for making any regulatory filings with respect to its products that incorporate our MRI-safety technologies. Boston Scientific will control the timing and manner of any regulatory filing, and will be responsible for the costs associated with any regulatory filing. We do not anticipate that we will be able to influence the process or timing in any meaningful way. In the United States, we believe that any Boston Scientific product incorporating our MRI-safety technologies will be a Class III medical device and require a PMA submission.
Our Strategy
Our key objective is to develop and commercialize medical systems to enable minimally invasive surgical procedures to be performed under direct, intra-procedural MRI guidance. Key elements of our strategy to achieve this objective are to:
| • | Maximize installation and adoption of our ClearPoint system. We are focusing our marketing efforts on key physicians and hospitals to adopt use of our ClearPoint system for general neurological interventional procedures. Our strategy is to convince those physicians that our ClearPoint system offers a better procedural solution to their patients. With the physicians serving as our internal champions, we will work with the physicians to encourage hospitals to install our ClearPoint system in their existing MRI suites. In hospitals where our ClearPoint system has been installed, we will focus on selling our disposable components to generate recurring revenue. |
| • | Continue development of the ClearTrace system with Siemens. We will continue to co-develop the ClearTrace system with Siemens. Together, we will work to generate awareness among leading physicians of the benefits of an MRI-guided approach to cardiac ablation for the treatment of cardiac arrhythmias, such as atrial fibrillation. Upon regulatory approval, we will work with Siemens to promote installation of the MRI software and our reusable components for the ClearTrace system within Siemens’ MRI customer base. In hospitals where the ClearTrace system has been installed, we will focus on selling our disposable components to generate recurring revenue. |
| • | Pursue SafeLead Development Program with Boston Scientific. We will continue collaboration with Boston Scientific with respect to the incorporation of our MRI-safety technologies into Boston Scientific’s implantable leads for cardiac and neurological applications. |
| • | Build upon our core technologies to continue to develop MRI-based products. Our research and development efforts to date have focused on developing novel MRI-related technologies. We have significant intellectual property protection in this particular area. As the field of MRI-guided interventions grows, we intend to develop future enhancements to our ClearPoint system and the ClearTrace system, as well as researching opportunities for new products. |
71
Licenses and Collaborative Relationships
In addition to our internally-developed technologies and devices, we have established and intend to continue to pursue licenses and collaborative relationships with medical device companies and academic institutions to further the development and commercialization of our core technologies and product platforms. Our current licenses and collaborative relationships are discussed below. The underlying agreements are filed as exhibits to the registration statement of which this prospectus is a part.
Siemens
In May 2009, we entered into a cooperation and development agreement with Siemens to develop the hardware and MRI software systems for MRI-guided, catheter-based ablation to treat cardiac arrhythmias, such as atrial fibrillation. Under this agreement, Siemens is responsible for developing the software in accordance with our specifications, and we are responsible for developing the catheters and other hardware, other than the MRI scanner and workstation, necessary for the MRI-guided cardiac ablation procedures and for the integration work necessary to combine the software, catheters and other hardware to create the ClearTrace system. We are obligated to pay Siemens up to approximately $2,500,000 in milestone-based payments associated with Siemens’ successful development of the software. These payments started in the second quarter of 2009 and will continue through the third quarter of 2011. Once the software is commercially available, Siemens will pay to us a fixed amount for each software license sold by Siemens until we recoup our investment. The term of the agreement will expire once (i) all software, catheter and other hardware development and integration work has been successfully completed, (ii) requisite regulatory clearances or approvals have been obtained in at least the United States, Canada and Europe, and (iii) the product has been clinically released in at least the United States, Canada and Europe. The agreement provides for exclusivity for a period of five years following the date of regulatory clearance and/or approval, determined on a country-by-country basis. During the exclusivity period, Siemens may not market or offer software that is intended to work with a third party’s catheters to conduct an MRI-guided cardiac ablation procedure, and we may not sell or offer any catheters that are intended to be used with an MRI scanner manufactured by a third party to conduct an MRI-guided cardiac ablation procedure. For two years after the exclusivity period ends, neither we nor Siemens may enter into an agreement or relationship with a third party that excludes or prevents the use of our devices with Siemens’ MRI systems, and vice versa, in the field of MRI-guided cardiac ablation procedures. Prior to or upon expiration of the term of the cooperation and development agreement, we anticipate entering into a separate sales and marketing agreement with Siemens.
Boston Scientific
We have entered into development and license agreements with affiliates of Boston Scientific. We are working together with Boston Scientific in the application of our technologies for potential use in Boston Scientific’s active implantable devices.
Neuro. In December 2005, we entered into a development agreement and license agreement with Boston Scientific in the neurological field:
| • | System and Lead Development and Transfer Agreement. We are working jointly with Boston Scientific to design and develop MRI-compatible and MRI-safe implantable leads for neurological applications, such as implantable deep brain stimulation leads. Under the development agreement, we could receive up to $1,600,000 in future milestone-based payments associated with successful development and regulatory approval of the leads. In addition, we could receive over $500,000 in incentive payments for incremental development work Boston Scientific may request. However, if our development milestones are not completed by December 31, 2012, the development agreement requires us to repay Boston Scientific certain amounts, including any milestone payments previously paid to us by Boston Scientific under this agreement and any patent prosecution costs incurred by Boston Scientific with respect to the intellectual property licensed to Boston Scientific pursuant to the technology license agreement described below. As of March 31, 2010, the potential obligation to Boston Scientific was approximately $750,000, plus costs incurred by Boston Scientific in prosecuting the licensed intellectual property. |
72
| • | Technology License Agreement. Under the license agreement, we granted Boston Scientific an exclusive worldwide license with respect to certain of our owned or licensed intellectual property in the neurological field to make, use, import, lease and sell neuro-related leads, neuro-related lead extensions, and neuro-related lead-type devices, such as implantable pulse generators. The license included a sublicense of applicable intellectual property that we licensed from Johns Hopkins. Boston Scientific has agreed to pay us royalties on net sales of products that are covered by a licensed patent; however, Boston Scientific has no obligation to include the licensed intellectual property in its products or product candidates. Pursuant to the system and lead development and transfer agreement described above, Boston Scientific is responsible for patent prosecution of the licensed intellectual property and the payment of costs associated with patent prosecution. |
Cardiac. In March 2008, we entered into a development agreement and license agreement with Boston Scientific in the field of implantable medical leads for cardiac applications.
| • | Development Agreement. Under the development agreement, we are working jointly with Boston Scientific to assess the feasibility of and, upon successful completion of feasibility studies, to design and develop three different MRI-compatible, MRI-safe implantable leads, a lead intended for bradycardia, a lead intended for tachycardia and a lead intended for heart failure. We could receive up to $20,000,000 in future milestone-based payments associated with the successful development and regulatory approval of those implantable lead types. No earned milestone payments will be made unless and until the applicable lead is covered by an issued patent licensed to Boston Scientific pursuant to the technology license agreement described below. The development agreement is scheduled to expire upon FDA approval of a design for each of the three different lead types. However, Boston Scientific has the one-time option, within 60 days after successful completion of the first lead feasibility study, to cease further development and to terminate the development agreement. |
| • | Technology License Agreement. Under the license agreement, we granted Boston Scientific an exclusive worldwide license with respect to certain of our owned or licensed intellectual property in the field of implantable medical leads for cardiac applications to make, have made, use, promote, market, import, distribute, lease, sell, offer for sale and commercialize products in that particular field of use. The license included a sublicense of applicable intellectual property that we licensed from Johns Hopkins. We received licensing fees of $13,000,000 in 2008. Boston Scientific has also agreed to pay us royalties on net sales of products that are covered by a licensed patent; however, Boston Scientific has no obligation to include our licensed intellectual property in its products or product candidates. Boston Scientific is responsible for patent prosecution of the licensed intellectual property and the payment of costs associated with patent prosecution. If Boston Scientific elects to exercise its termination option under the development agreement described above, the license we granted Boston Scientific will automatically become non-exclusive with respect to some intellectual property, other intellectual property will be removed the scope of the license and revert to us, and Boston Scientific will not be obligated to pay us future royalties or sublicense revenues based on sales of products covered by any issued patent that remains subject to the non-exclusive license. |
University of California, San Francisco
In August 2007, we entered into a research agreement with the University of California, San Francisco, or UCSF, which has been amended from time to time since that date. Under our agreement, UCSF personnel are conducting research activities relating to interventional MRI guidance for the performance of certain minimally invasive neurological procedures, including an assessment of the safety and clinical efficacy of such procedures. We agreed to make periodic payments to UCSF to fund its research. In addition, to further support UCSF’s research activities, we agreed to make an in-kind contribution to UCSF of some of the reusable components of our ClearPoint system and other MRI-related equipment. In return for supporting UCSF’s research, we received the first option to license, exclusively or non-exclusively, any intellectual property conceived or created by UCSF personnel under the research project. Our agreement with UCSF will terminate November 1, 2011, unless UCSF and we agree to extend the term.
73
The University of Utah
In July 2007, we entered into a research agreement with The University of Utah, or Utah, which has been amended from time to time since that date. Under the agreement, Utah personnel are conducting research activities and experiments to develop knowledge, techniques, methods and technologies related to MRI-guided cardiac ablation, including a specific focus on MRI-guided cardiac ablation to treat atrial fibrillation. We agreed to make periodic payments to Utah to fund its research activities. In return, Utah granted us a non-exclusive, worldwide license to any intellectual property created or conceived by Utah personnel in the performance of the research. In addition, we also received the first option to license exclusively any such intellectual property. Our agreement with Utah will terminate December 31, 2010, unless Utah and we agree to extend the term.
The Johns Hopkins University
We have in place five exclusive license agreements with Johns Hopkins. For additional information regarding these licenses, see “Business – Intellectual Property.”
Sales and Marketing
Commercializing our ClearPoint system involves marketing to:
| • | physicians, who care for patients suffering from neurological disorders, including neurosurgeons, who perform the neurological procedures, and neurologists, who interact with patients prior to and following the therapy and who refer patients to therapy; |
| • | hospitals involved in the treatment of neurological disorders and the opinion leaders at these hospitals; and |
| • | patients who suffer from neurological disorders. |
There are approximately 3,500 neurosurgeons in the United States. Similar to many fields of medicine, some neurosurgeons elect to focus on a particular specialty within the neurological field. For example, some neurosurgeons focus their practice on spine surgeries, others more on open craniotomy surgeries and others more on minimally invasive approaches, such as functional neurosurgery. We believe our ClearPoint system is most applicable to those functional neurosurgeons, of whom there are approximately 300 in the United States. Part of our business objective is to encourage adoption of our ClearPoint system by functional neurosurgeons by securing placement of our system within their hospitals. We believe that our ClearPoint system represents an attractive platform for the functional neurosurgery team within a hospital to perform various general neurological interventions.
Presently, our sales and marketing efforts for our ClearPoint system are being coordinated primarily by our Vice President, Sales, our Vice President, Product Management and our two Clinical Engineering Managers, one of whom is located on the east coast of the United States and the other of whom is located on the west coast of the United States. We expect to expand our existing sales and marketing capabilities by building a small, highly focused sales force to market our ClearPoint system products in the United States. Given the number of functional neurosurgeons in the United States, we believe a small, direct sales force will be sufficient and effective for us to reach our target market. We recently hired Mr. John Keane to serve as our Vice President, Sales and in the future anticipate hiring a limited number of additional sales people for the ClearPoint sales force. We have not finalized a sales and marketing plan to commercialize our ClearPoint system outside the United States; however, any such plan could involve the establishment of collaborations with third-parties.
Given the stage of development of the ClearTrace system, we have not developed a sales and marketing plan to commercialize ClearTrace either inside or outside the United States. We will not develop a sales and marketing plan to commercialize any of our SafeLead Development Program technologies as Boston Scientific is in control of the commercialization of those technologies for its implantable medical leads.
74
Research and Development
Continued innovation through research and development is critical to our future success. As of April 30, 2010, our research and development team, which is based primarily in our Irvine, California facility, consisted of 11 employees. We have assembled an experienced team with recognized expertise in both the development of medical devices and advanced MRI technologies, including interventional MRI microcoils and catheters. We believe that our current research and development team is sufficient for our current needs; however, we may increase the size of our team depending on the progress of our ongoing research and development efforts.
Our principal research and development goals are:
| • | to complete development of the ClearTrace system in cooperation with Siemens; |
| • | to continue to enhance our ClearPoint system; and |
| • | to provide technical support and expertise in the area of MRI safety to Boston Scientific under our SafeLead Development Program. |
We have historically spent a significant portion of our capital resources on research and development. Our research and development expenses were approximately $2,099,000, $4,258,000 and $6,068,000 for the years ended December 31, 2007, 2008 and 2009, respectively. Our research and development expenses were approximately $1,747,000 for the three months ended March 31, 2010.
Manufacturing and Assembly
Our ClearPoint system includes off-the-shelf components, custom-made components produced to our proprietary specifications by various third parties and components that we assemble in our Irvine, California facility. We use third parties to manufacture these components to utilize their individual expertise, minimize our capital investment and help control costs. We purchase most custom-made components of our ClearPoint system from a single source due to quality considerations, lower costs and constraints resulting from regulatory requirements; however, we believe alternative sources are available, if needed. Generally, we purchase our components through purchase orders and do not have long-term contracts with most of our suppliers.
Our Irvine, California facility is structured to complete component processing, final assembly, packaging and distribution activities for our ClearPoint system. The assembly process is performed in a controlled environment as required for medical device assembly by applicable regulation. Our operations are subject to extensive regulation by the FDA under its QSR, which requires that manufacturers have a quality management system for the design and production of medical devices. In addition, in the event we expand our business outside the United States, we will also become subject to international regulatory requirements.
Our Irvine, California facility is FDA-registered, and we believe it is compliant with the FDA’s QSR. We have instituted a quality management system, under which we have established policies and procedures that control and direct our operations with respect to design, procurement, manufacture, inspection, testing, installation, data analysis, training and marketing. We review and internally audit our compliance with these policies and procedures, which provides a means for continued evaluation and improvement. As required by our quality management system, we undertake an assessment and qualification process for each third-party manufacturer or supplier that we use. Typically, our third-party manufacturers and suppliers are certified to ISO standard 9001 and/or 13485. We also periodically perform audit procedures on our third-party manufacturers and suppliers to monitor their activities for compliance with our quality management system. Our facility and the facilities of the third-party manufacturers and suppliers we use are subject to periodic inspections by regulatory authorities, including the FDA and other governmental agencies.
75
Intellectual Property
We believe that in order to maintain a competitive advantage in the marketplace, we must develop and maintain the proprietary aspects of our technologies. We rely on a combination of patent, trademark, trade secret, copyright and other intellectual property rights and measures to protect our intellectual property.
Our patent portfolio includes rights to patents and patent applications that we own, whether wholly-owned or co-owned, or license from others. We seek patent protection in the United States and internationally for our products and technologies where and when we believe it is appropriate. United States patents are granted generally for a term of 20 years from the earliest effective priority date of the patent application. The actual protection afforded by a foreign patent, which can vary from country to country, depends on the type of patent, the scope of its claims and the availability of legal remedies in the country.
We also rely on other forms of intellectual property rights and measures, including trade secrets and nondisclosure agreements, to maintain and protect proprietary aspects of our products and technologies. We require our employees and consultants to execute confidentiality agreements in connection with their employment or consulting relationships with us. We also require our employees and consultants to disclose and assign to us all inventions conceived during the term of their employment or engagement while using our property or which relate to our business.
Owned Patents and Patent Applications
As of April 30, 2010, we wholly owned eight issued United States patents (including one design patent), 25 pending United States patent applications (including five provisional applications), one issued foreign patent and 38 pending foreign patent applications (including seven Patent Cooperation Treaty applications). In addition, as of April 30, 2010, we co-owned with third-parties a total of four issued United States patents, nine pending United States patent applications, one issued foreign patent and 22 pending foreign patent applications (including one Patent Cooperation Treaty application).
Of those co-owned patents and patent applications, as of April 30, 2010, three issued United States patents, one pending United States patent application, one issued foreign patent and four pending foreign patent applications were co-owned by us and Johns Hopkins, one issued United States patent, seven pending United States patent applications and 17 pending foreign patent applications (including one Patent Cooperation Treaty application) were co-owned by us and Boston Scientific, and one pending United States patent application and one pending foreign patent application were co-owned by us and other third parties.
We have licensing and cross-licensing arrangements in place with Boston Scientific with respect to the patent and patent applications we co-own with them. As a result of those arrangements, we have exclusive rights to all fields outside neuromodulation and implantable medical leads for cardiac applications, and we have licensed the fields of neuromodulation and implantable medical leads for cardiac applications to Boston Scientific. Our owned, issued patents expire at various dates beginning in 2020.
Patents and Patent Applications Licensed from Third-Parties
As of April 30, 2010, we had licensed rights to 11 United States and 15 foreign third-party issued patents, and we had licensed rights to nine United States and 12 foreign third-party pending patent applications. Our licensed, issued patents expire at various dates beginning in 2015.
License Arrangements
Our license arrangements are discussed below. The underlying agreements are filed as exhibits to the registration statement of which this prospectus is a part.
License Arrangements with The Johns Hopkins University
Our principal licensing arrangement is with Johns Hopkins. Shortly following our formation in 1998, we entered into a license agreement with Johns Hopkins pursuant to which we obtained an exclusive, worldwide license to a number of technologies owned by Johns Hopkins relating to devices, systems and methods for
76
performing MRI-guided interventions, such as MRI-guided cardiac ablation procedures. The field of use for this exclusive license covers diagnostic or therapeutic methods, processes or devices using an intravascular, intralumen or intratissue miniature magnetic resonance coil detection probe. We are obligated to pay Johns Hopkins an annual maintenance fee, and we are also obligated to pay a royalty to Johns Hopkins based on the sale of products or provision of services covered by licensed patent. To the extent we sublicense any licensed intellectual property to a third-party, we agreed to pay Johns Hopkins a percentage of revenue we receive as a result of the sublicense. Under our license agreements with Boston Scientific, we sublicensed intellectual property that is licensed from Johns Hopkins. Therefore, we are obligated to pay Johns Hopkins a percentage of any revenue we receive from sales by Boston Scientific of products covered by a sublicensed patent. This license agreement with Johns Hopkins will terminate upon the expiration of the last to expire of the licensed patents.
In December 2006, we entered into a second license agreement with Johns Hopkins under which we obtained an exclusive, worldwide license to certain MRI-safety technologies owned by Johns Hopkins. Under the agreement, we are obligated to pay a royalty to Johns Hopkins based on the sale of products or provision of services covered by a licensed patent, subject to a minimum annual payment. Likewise, to the extent we sublicense any intellectual property to a third party, we agreed to pay Johns Hopkins a percentage of revenue we receive as a result of the sublicense. Under our license agreements with Boston Scientific, we sublicensed intellectual property that is licensed from Johns Hopkins. Therefore, we are obligated to pay Johns Hopkins a percentage of any revenue we receive from sales by Boston Scientific of products covered by a sublicensed patent. This license agreement with Johns Hopkins will terminate upon the expiration of the last to expire of the licensed patents.
We entered into three additional exclusive license agreements with Johns Hopkins in June 2008 as described below. Our development efforts with respect to the technologies we licensed under those agreements are at a very early stage.
| • | Under the first agreement, we obtained an exclusive, worldwide license to certain catheter technology owned by Johns Hopkins. Under this agreement, we are obligated to pay a royalty to Johns Hopkins based on the sale of products or provision of services incorporating the licensed technology and a license fee. Likewise, to the extent we sublicense any licensed technology to a third party, we agreed to pay Johns Hopkins a percentage of revenue we receive as a result of a sublicense of the licensed technology. This license agreement with Johns Hopkins will terminate upon the expiration of the last licensed patent or, if no patent issues, on June 30, 2028. |
| • | Under the second agreement, we obtained an exclusive, worldwide license to certain technology owned by Johns Hopkins relating to catheter-based MRI probes. Under this agreement, we are obligated to pay a royalty to Johns Hopkins based on the sale of products or provision of services incorporating the licensed technology and a contingent license fee in the event a United States patent issues for the licensed technology. Likewise, to the extent we sublicense any licensed technology to a third party, we agreed to pay Johns Hopkins a percentage of revenue we receive as a result of a sublicense of the licensed technology. This license agreement with Johns Hopkins will terminate upon the expiration of the last licensed patent or, if no patent issues, on June 30, 2028. In addition, Johns Hopkins has the option to terminate the license in the event that a commercial sale of a licensed product or a licensed service does not occur by June 30, 2012. |
| • | Under the third agreement, we obtained an exclusive, worldwide license to certain technology owned by Johns Hopkins to measure the amount of radio frequency absorption in the human body during an MRI scan. Under this agreement, we are obligated to pay a royalty to Johns Hopkins based on the sale of products or provision of services incorporating the licensed technology. Likewise, to the extent we sublicense any licensed technology to a third party, we agreed to pay Johns Hopkins a percentage of revenue we receive as a result of a sublicense of the licensed technology. This license agreement with Johns Hopkins will terminate upon the expiration of the last licensed patent or, if no patent issues, on June 30, 2028. |
77
License Arrangements with Cedara Software Corp.
In July 2007, we entered into a master service and license agreement with Cedara Software Corp. (d/b/a Merge OEM), or Cedara, for Cedara to develop on our behalf, based on our detailed specifications, a customized software solution for our ClearPoint system. Cedara is in the business of providing software development and engineering services on a contract basis to a number of companies. In developing our ClearPoint system software, Cedara utilized certain of its own pre-existing software code. Under our agreement with Cedara, we received a non-exclusive, worldwide license to that code as an integrated component of our ClearPoint system software. In return, we agreed to pay Cedara a license fee for each copy of our ClearPoint system software that we distribute. Except for Cedara’s pre-existing software code, the work performed by Cedara was a “work-made-for-hire” and we exclusively own our ClearPoint system software. The agreement provides for annual minimum licensing fees. Our license from Cedara continues through July 2015, absent a mutual extension of the license term. If necessary, we could replace the licensed Cedara code.
License Arrangements with the National Institutes of Health
In April 2009, we entered into a patent license agreement with the National Institutes of Health, or NIH, that covers techniques for three dimensional renderings of the patient’s anatomy from MRI data in real time. The techniques underlying this patent may be used in the development of the ClearTrace system. Under the terms of this agreement, we have a non-exclusive license to a pending United States patent application within the field of devices and systems for MRI-guided medical procedures. Our licensed territory includes Australia, Canada, China, Europe, Israel, Japan and the United States, although there is no patent or patent application pending for the licensed intellectual property outside the United States. Pursuant to this agreement, we are obligated to make royalty payments to NIH based on the sale of products and the practice of processes covered by the licensed intellectual property, whether by us or any sublicensee. In addition, NIH is entitled to receive a single milestone payment in the event we receive a regulatory clearance or approval of a product or process covered by the licensed intellectual property.
Competition
General
The length of time required for products to be developed and to receive regulatory and, in some cases, reimbursement clearance or approval is an important competitive factor. However, even if we are successful in obtaining regulatory clearances or approvals, the medical device industry is characterized by rapid and significant technological change. Thus, the development by others of new treatment methods, including novel drugs, medical devices or surgical techniques could render our product candidates non-competitive or obsolete. As a result, product development involves a high degree of risk and there can be no assurance that our current or new product development efforts will result in any commercially successful products.
ClearPoint System
Our success depends on convincing hospitals, neurosurgeons, neurologists and patients to utilize our ClearPoint system. Currently, we are not aware of any other company that offers a direct MRI-guided stereotactic system for neurological interventions, although two companies, Monteris Medical Inc. and Visualase, Inc., do offer devices for laser ablation under direct MRI guidance. However, we do face competition from companies, such as BrainLAB AG, Elekta AB, FHC Inc. and Medtronic, Inc., which offer instruments and systems for use in conventional stereotactic neurological procedures, such as surgical navigation workstations and frame-based and frameless stereotactic systems. Additionally, we could also face competition from other medical device and pharmaceutical companies that have the technology, experience and capital resources to develop alternative therapy methods, including MRI-guided technologies. Many of our competitors have substantially greater financial, manufacturing, marketing and technical resources than we have.
78
ClearTrace System
Our success depends on convincing hospitals, physicians and patients to utilize the ClearTrace system for performing cardiac ablation procedures. While we are not aware of any companies that currently offer a direct MRI-guided cardiac ablation system, companies such as GE Healthcare, Imricor Medical Systems, Inc. and Philips Healthcare may be in the process of developing such a system. We are not aware of any potential competitive advantages or disadvantages relative to any such system that may be under development; however, if any of these companies develops, obtains regulatory clearance or approval and achieves commercial success for a direct MRI-guided cardiac ablation system, the ClearTrace system could be rendered non-competitive or obsolete.
We also will face competition from companies who are engaged in the development and marketing of conventional catheter-based cardiac ablation systems and devices. These products include mapping systems using contact mapping, single-point spatial mapping and non-contact, multi-site electrical mapping technologies and ablation systems using radio frequency, ultrasound, laser and cryoablation technologies. These products evolve rapidly, and their manufacturers are constantly attempting to make them easier to use or more efficacious in performing procedures. Today, the vast majority of minimally invasive catheter-based cardiac ablation procedures are performed with these products. Because these products are currently in use while the ClearTrace system remains under development, physician preferences will have to shift for the ClearTrace system to gain market acceptance. We believe that the primary factors which will drive physician preference will be the relative success rates and ease of the procedure for physicians with respect to the ClearTrace system compared to the alternative technologies available.
We are aware of two companies, Hansen Medical, Inc. and Stereotaxis, Inc., that market systems to remotely control catheters during interventional cardiac ablation and other procedures using either robotic or magnetic steering. The nature of these systems potentially could provide better control over the catheter compared to manual manipulation by the physician; however, these systems do not provide the physician with detailed intra-procedural visualization of the cardiac tissue. Also, other manufacturers are attempting to market devices that access the exterior of the heart wall through an endoscopic surgical technique called thoracoscopy to treat atrial fibrillation. Because this procedure was developed recently, the clinical advantages and disadvantages of this approach compared to a catheter-based approach inside the heart have not been established. Therefore, we are not aware of any competitive advantages or disadvantages of this procedure relative to the anticipated ClearTrace system procedure.
Additionally, we will face competition from large companies who are engaged in the development and marketing of products for other treatments of cardiac arrhythmias, such as atrial fibrillation. Their products include drugs, implantable devices, such as implantable defibrillators and pacemakers, and the devices used in open-heart surgery. While both current drug therapy and implantable cardiac devices can be effective in treating the symptoms of atrial fibrillation, they do not provide a cure for the underlying disease. Open-heart surgery, such as the Cox-Maze procedure, can provide a cure for atrial fibrillation and reported success rates have been very high; however, it is an invasive surgical procedure that is traumatic to the patient, very expensive and typically associated with long hospital stays and recovery times.
Many of our potential competitors have an established presence in the field of cardiac electrophysiology, including cardiac ablation, such as Biosense Webster Inc., a division of Johnson & Johnson, Boston Scientific, Medtronic, Inc. and St. Jude Medical, Inc. These potential competitors have substantially greater financial and other resources than we do, including larger research and development staffs and more experience and greater capabilities in conducting research and development activities, testing products in clinical trials, obtaining regulatory clearances or approvals, and manufacturing, marketing and distributing products.
SafeLead Development Program
Because manufacturers studies have indicated that cardiologists identify “MRI-compatibility” of implantable medical leads as one of the main features that would drive a change in brand preference, we believe that other medical device companies are developing proprietary MRI-safe lead designs. For example, in some
79
European countries, Medtronic is currently marketing a device called an “MR-conditional” pacemaker system, including a pacemaker and implantable leads, which is designed for use with MRI under certain conditions specified in product labeling. This product is relatively new to the market and therefore we are not able to determine the degree to which it has achieved market acceptance. We are working together with Boston Scientific to incorporate our MRI-safety technologies into Boston Scientific’s implantable leads for cardiac and neurological applications. These development efforts for the SafeLead Development Program are ongoing and therefore we are unable to describe the way in which the Boston Scientific implantable leads will differ from the Medtronic leads. We believe that any Boston Scientific device developed from the SafeLead Development Program will compete with similar devices that may be commercialized by other manufacturers.
Regulatory Requirements of the United States Food and Drug Administration
Our research, development and clinical programs, as well as our manufacturing and marketing operations, are subject to extensive regulation in the United States and other countries. Most notably, all of our products sold in the United States are subject to regulation as medical devices under the federal Food Drug and Cosmetic Act, as implemented and enforced by the FDA. The FDA governs the following activities that we perform or that are performed on our behalf, to ensure that the medical products we manufacture, promote and distribute domestically or exported internationally are safe and effective for their intended uses:
| • | product design, preclinical and clinical development and manufacture; |
| • | product premarket clearance and approval; |
| • | product safety, testing, labeling and storage; |
| • | record keeping procedures; |
| • | product marketing, sales and distribution; and |
| • | post-marketing surveillance, complaint handling, medical device reporting, reporting of deaths, serious injuries or device malfunctions and repair or recall of products. |
FDA Premarket Clearance and Approval Requirements
Unless an exemption applies, each medical device we wish to commercially distribute in the United States will require either premarket notification, or 510(k) clearance, or approval of a premarket approval application, or PMA, from the FDA. The FDA classifies medical devices into one of three classes. Class I devices, considered to have the lowest risk, are those for which safety and effectiveness can be assured by adherence to the FDA’s general regulatory controls for medical devices, which include compliance with the applicable portions of the FDA’s QSR, facility registration and product listing, reporting of adverse medical events, and appropriate, truthful and non-misleading labeling, advertising, and promotional materials (General Controls). Class II devices are subject to the FDA’s General Controls, and any other special controls as deemed necessary by the FDA to ensure the safety and effectiveness of the device (Special Controls). Manufacturers of most Class II and some Class I devices are required to submit to the FDA a premarket notification under Section 510(k) of the FDCA requesting permission to commercially distribute the device. This process is generally known as 510(k) clearance. Devices deemed by the FDA to pose the greatest risks, such as life-sustaining, life-supporting or implantable devices, or devices that have a new intended use, or use advanced technology that is not substantially equivalent to that of a legally marketed device, are placed in Class III, requiring approval of a PMA.
510(k) Clearance Pathway
When a 510(k) clearance is required, we will be required to submit a 510(k) application demonstrating that our proposed device is substantially equivalent to a previously cleared 510(k) device or a device that was in commercial distribution before May 28, 1976 for which the FDA has not yet called for the submission of PMAs. By regulation, the FDA is required to clear or deny a 510(k) premarket notification within 90 days of submission of the application. As a practical matter, clearance may take longer. The FDA may require further information, including clinical data, to make a determination regarding substantial equivalence.
80
Once filed, the FDA has 90 days in which to review the 510(k) application and respond. Typically, the FDA’s response after reviewing a 510(k) application is a request for additional data or clarification. Depending on the complexity of the application and the amount of data required, the process may be lengthened by several months or more. If additional data, including clinical data, are needed to support our claims, the 510(k) application process may be significantly lengthened.
If the FDA issues an order declaring the device to be Not Substantially Equivalent, or NSE, the device is placed into a Class III or PMA category. At that time, a company can request a de novo classification of the product. De novo generally applies where there is no predicate device and the FDA believes the device is sufficiently safe so that no PMA should be required. The request must be in writing and sent within 30 days from the receipt of the NSE determination. The request should include a description of the device, labeling for the device, reasons for the recommended classification and information to support the recommendation. The de novo process has a 60 day review period. If the FDA classifies the device into Class II, a company will then receive an approval order to market the device. This device type can then be used as a predicate device for future 510(k) submissions. However, if the FDA subsequently determines that the device will remain in the Class III category, the device cannot be marketed until the company has obtained an approved PMA. If we are required to follow a de novo process, an additional 60 to 90 days or more will be added on to the original 90 days required for the initial 510(k) review.
Any modification to a 510(k)-cleared device that would constitute a major change in its intended use, or any change that could significantly affect the safety or effectiveness of the device, requires a new 510(k) clearance and may even, in some circumstances, require a PMA, if the change raises complex or novel scientific issues or the product has a new intended use. The FDA requires every manufacturer to make the determination regarding the need for a new 510(k) submission in the first instance, but the FDA may review any manufacturer’s decision. If the FDA were to disagree with any of our determinations that changes did not require a new 510(k) submission, it could require us to cease marketing and distribution and/or recall the modified device until 510(k) clearance or PMA approval is obtained. If the FDA requires us to seek 510(k) clearance or PMA approval for any modifications, we may be required to cease marketing and/or recall the modified device, if already in distribution, until 510(k) clearance or PMA approval is obtained and we could be subject to significant regulatory fines or penalties.
PMA Approval Pathway
A PMA must be submitted to the FDA if the device cannot be cleared through the 510(k) process, or is not otherwise exempt from the FDA’s premarket clearance and approval requirements. A PMA must generally be supported by extensive data, including, but not limited to, technical, preclinical, clinical trials, manufacturing and labeling, to demonstrate to the FDA’s satisfaction the safety and effectiveness of the device for its intended use. During the review period, the FDA will typically request additional information or clarification of the information already provided. Also, an advisory panel of experts from outside the FDA may be convened to review and evaluate the application and provide recommendations to the FDA as to the approvability of the device. The FDA may or may not accept the panel’s recommendation. In addition, the FDA will generally conduct a pre-approval inspection of our or our third-party manufacturers’ or suppliers’ manufacturing facility or facilities to ensure compliance with the QSR. Once a PMA is approved, the FDA may require that certain conditions of approval, such as conducting a post market clinical trial, be met.
New PMAs or PMA supplements are required for modifications that affect the safety or effectiveness of the device, including, for example, certain types of modifications to the device’s indication for use, manufacturing process, labeling and design. PMA supplements often require submission of the same type of information as a PMA, except that the supplement is limited to information needed to support any changes from the device covered by the original PMA and may not require as extensive clinical data or the convening of an advisory panel. We have not submitted any of our product candidates for a PMA approval. However, we may in the future develop devices that will require the approval of a PMA, or seek to add new indications for use of existing
81
products that require the approval of a PMA. There is no guarantee that the FDA will grant PMA approval of these specific indications for use or for our future products and failure to obtain necessary approvals for our future products would adversely affect our ability to grow our business.
Clinical Trials
Clinical trials are generally required to support a PMA application and are sometimes required for 510(k) clearance. Such trials generally require an application for an investigational device exemption, or IDE, which is approved in advance by the FDA for a specified number of patients and study sites, unless the product is deemed a non-significant risk device eligible for more abbreviated IDE requirements. A significant risk device is one that presents a potential for serious risk to the health, safety, or welfare of a patient and either is implanted, used in supporting or sustaining human life, substantially important in diagnosing, curing, mitigating, or treating disease or otherwise preventing impairment of human health, or otherwise presents a potential for serious risk to a subject. Clinical trials are subject to extensive monitoring, recordkeeping and reporting requirements. Clinical trials must be conducted under the oversight of an institutional review board, or IRB, for the relevant clinical trial sites and must comply with FDA regulations, including, but not limited to, those relating to good clinical practices. To conduct a clinical trial, we also are required to obtain the patient’s informed consent in a form and substance that complies with both FDA requirements and state and federal privacy and human subject protection regulations. We, the FDA or the IRB could suspend a clinical trial at any time for various reasons, including a belief that the risks to study subjects outweigh the anticipated benefits. Even if a trial is completed, the results of clinical testing may not adequately demonstrate the safety and efficacy of the device or may otherwise not be sufficient to obtain FDA clearance or approval to market the product in the United States. Similarly, in Europe, the clinical study must be approved by a local ethics committee and in some cases, including studies with high-risk devices, by the ministry of health in the applicable country.
Pervasive and Continuing Regulation
After a device is placed on the market, numerous regulatory requirements continue to apply. In addition to the requirements below, the MDR regulations require that we report to the FDA any incident in which our products may have caused or contributed to a death or serious injury or in which our product malfunctioned and, if the malfunction were to recur, would likely cause or contribute to death or serious injury. Additional regulatory requirements include:
| • | product listing and establishment registration, which helps facilitate FDA inspections and other regulatory action; |
| • | QSR, which requires manufacturers, including third-party manufacturers, to follow stringent design, testing, control, documentation and other quality assurance procedures during all aspects of the design and manufacturing process; |
| • | labeling regulations and FDA prohibitions against the promotion of products for uncleared, unapproved or off-label use or indication; |
| • | clearance of product modifications that could significantly affect safety or effectiveness or that would constitute a major change in intended use of one of our cleared devices; |
| • | approval of product modifications that affect the safety or effectiveness of one of our approved devices; |
| • | post-approval restrictions or conditions, including post-approval study commitments; |
| • | post-market surveillance regulations, which apply, when necessary, to protect the public health or to provide additional safety and effectiveness data for the device; |
| • | the FDA’s recall authority, whereby it can ask, or under certain conditions order, device manufacturers to recall from the market a product that is in violation of governing laws and regulations; |
82
| • | regulations pertaining to voluntary recalls; and |
| • | notices of corrections or removals. |
As a manufacturer, we are subject to announced and unannounced inspections by the FDA to determine our compliance with FDA’s QSR and other regulations. We have not yet been inspected by the FDA. We believe that we are in compliance with QSR and other regulations.
Advertising and promotion of medical devices, in addition to being regulated by the FDA, are also regulated by the United States Federal Trade Commission, or FTC, and by state regulatory and enforcement authorities. Recently, promotional activities for FDA-regulated products of other companies have been the subject of enforcement action brought under healthcare reimbursement laws and consumer protection statutes. In addition, under the federal Lanham Act and similar state laws, competitors and others can initiate litigation relating to advertising claims. In addition, we are required to meet regulatory requirements in countries outside the United States, which can change rapidly with relatively short notice. If the FDA determines that our promotional materials or training constitutes promotion of an unapproved or uncleared use, it could request that we modify our training or promotional materials or subject us to regulatory or enforcement actions, including the issuance of an untitled letter, a warning letter, injunction, seizure, civil fine or criminal penalty. It is also possible that other federal, state or foreign enforcement authorities might take action if they consider our promotional or training materials to constitute promotion of an unapproved use, which could result in significant fines or penalties under other statutory authorities, such as laws prohibiting false claims for reimbursement. In that event, our reputation could be damaged and adoption of the products would be impaired.
Failure by us or by our third-party manufacturers and suppliers to comply with applicable regulatory requirements can result in enforcement action by the FDA or other regulatory authorities, which may result in sanctions including, but not limited to:
| • | untitled letters, warning letters, fines, injunctions, consent decrees and civil penalties; |
| • | customer notifications or repair, replacement, refunds, recall, detention or seizure of our marketed products; |
| • | operating restrictions or partial suspension or total shutdown of production; |
| • | refusing or delaying requests for 510(k) clearance or PMA approvals of new products or modified products; |
| • | withdrawing 510(k) clearances or PMA approvals that have already been granted; |
| • | refusal to grant export approval for our marketed products; or |
| • | criminal prosecution. |
International Marketing Approvals
International sales of medical devices are subject to foreign government regulations, which vary substantially from country to country. The time required to obtain approval by a foreign country may be longer or shorter than that required for FDA clearance or approval, and the requirements may differ.
The European Union has adopted numerous directives and standards regulating the design, manufacture, clinical trials, labeling, and adverse event reporting for medical devices. Each European Union member state has implemented legislation applying these directives and standards at a national level. Other countries, such as Switzerland, have voluntarily adopted laws and regulations that mirror those of the European Union with respect to medical devices. Devices that comply with the requirements of the laws of the relevant member state applying the applicable European Union directive are entitled to bear a CE mark and, accordingly, can be distributed throughout the member states of the European Union and other countries that comply with or mirror those directives.
83
The method of assessing conformity with applicable regulatory requirements varies depending on the classification of the medical device, which may be Class I, Class IIa, Class IIb or Class III. Normally, the method involves a combination of self-assessment by the manufacturer of the safety and performance of the device, and a third-party assessment by a Notified Body, usually of the design of the device and of the manufacturer’s quality system. A Notified Body is a private commercial entity that is designated by the national government of a member state as being competent to make independent judgments about whether a device complies with applicable regulatory requirements. An assessment by a Notified Body in one country with the European Union is required in order for a manufacturer to commercially distribute the device throughout the European Union. In addition, compliance with ISO 13485 issued by the International Organization for Standardization, among other standards, establishes the presumption of conformity with the essential requirements for CE marking. Certification to the ISO 13485 standard demonstrates the presence of a quality management system that can be used by a manufacturer for design and development, production, installation and servicing of medical devices and the design, development and provision of related services.
We intend to apply for CE marking approval for sale of our ClearPoint system during 2010, and we believe the components of our ClearPoint system will fall into different device classifications, including Class III. We have engaged KEMA as the Notified Body for our CE marking approval process. The exact regulatory pathway will be the subject of discussions with KEMA. At this time, we are unable to accurately predict when, if ever, CE marking will be obtained, whether clinical trials will be required as part of the CE marking approval process or the regulatory requirements to which we would be subject after approval.
Healthcare Laws and Regulations
Third-Party Reimbursement
In the United States and elsewhere, healthcare providers that perform surgical procedures using medical devices such as ours generally rely on third-party payors, including governmental payors such as Medicare and Medicaid and private payors, to cover and reimburse all or part of the cost of the products. Consequently, sales of medical devices are dependent in part on the availability of reimbursement to the customer from third-party payors. The manner in which reimbursement is sought and obtained varies based upon the type of payor involved and the setting in which the product is furnished and utilized. In general, third-party payors will provide coverage and reimbursement for medically reasonable and necessary procedures and tests that utilize medical devices and may provide separate payments for the implanted or disposable devices themselves. Most payors, however, will not pay separately for capital equipment, such as our ClearPoint system. Instead, payment for the cost of using the capital equipment is considered to be covered as part of payments received for performing the procedure. In determining payment rates, third-party payors are increasingly scrutinizing the prices charged for medical products and services in comparison to other therapies. Our marketed products, and the procedures in which our marketed products will be used, may not be reimbursed by these third-party payors at rates sufficient to allow us to sell our marketed products on a competitive and profitable basis.
In addition, in many foreign markets, including the countries in the European Union, pricing of medical devices is subject to governmental control. In the United States, there have been, and we expect that there will continue to be, a number of federal and state proposals to limit payments by governmental payors for medical devices, and the procedures in which medical devices are used. While we cannot predict whether such legislative or regulatory proposals will be adopted, the adoption of such proposals could have a material adverse effect on our business, financial condition and profitability.
Medicare and Medicaid
The Medicare program is a federal health benefit program administered by CMS that covers and pays for certain medical care items and services for eligible elderly and certain disabled individuals, and individuals with end stage renal disease. The Medicaid program is a federal-state partnership under which states receive matching federal payments to fund healthcare services for the poor. Because some private commercial health insurers and
84
some state Medicaid programs may follow the coverage and payment policies for Medicare, Medicare’s coverage and payment policies are significant to our business. On July 30, 2008, CMS released a list of potential topics for national coverage determinations. This list included ablation for atrial fibrillation and specifically asked whether the evidence was adequate to demonstrate health benefits in patients who receive the procedure. On October 21, 2009, the Medicare Evidence Development and Coverage Advisory Committee, or MedCAC, held a meeting on the adequacy of the available evidence for catheter ablation for the treatment of atrial fibrillation. Although CMS has not formally opened a national coverage analysis on this topic, the agency clearly is interested in the clinical evidence of atrial fibrillation treatments and any national coverage decisions it makes could have a material effect on our potential business in this area.
Medicare coverage for the procedures in which our products would be used currently exists in the hospital inpatient setting, which falls under Part A of the Medicare program. Under Medicare Part A, Medicare reimburses acute care hospitals a prospectively determined payment amount for beneficiaries receiving covered inpatient services in an acute care hospital. This method of payment is known as the prospective payment system, or PPS. Under PPS, the prospective payment for a patient’s stay in an acute care hospital is determined by the patient’s condition and other patient data and procedures performed during the inpatient stay using a classification system known as DRGs. Payments also are adjusted to reflect regional variations in labor costs, indirect medical education expenses, payments for hospitals that treat a disproportionate share of poor patients, and other factors. As of October 1, 2007, CMS implemented a revised version of the DRG system that uses 745 Medicare Severity DRGs, or MS-DRGs, instead of the approximately 540 DRGs Medicare previously used. The MS-DRGs are intended to account more accurately for the patient’s severity of illness when assigning each patient’s stay to a payment classification. Medicare pays a fixed amount to the hospital based on the MS-DRG into which the patient’s stay is classified, regardless of the actual cost to the hospital of furnishing the procedures, items and services that the patient’s condition requires. Accordingly, acute care hospitals generally do not receive direct Medicare reimbursement under PPS for the specific costs incurred in purchasing medical devices. Rather, reimbursement for these costs is deemed to be included within the MS-DRG-based payments made to hospitals for the services furnished to Medicare-eligible inpatients in which the devices are utilized. For cases involving unusually high costs, a hospital may receive additional “outlier” payments above the pre-determined amount. In addition, there is a mechanism by which new technology services can apply to Medicare for additional payments above the pre-determined amount, although such requests have not been granted frequently.
Because PPS payments are based on predetermined rates and may be less than a hospital’s actual costs in furnishing care, acute care hospitals have incentives to lower their inpatient operating costs by utilizing products, devices and supplies that will reduce the length of inpatient stays, decrease labor or otherwise lower their costs. For each MS-DRG, a relative weight is calculated representing the average resources required to care for cases grouped in that particular MS-DRG relative to the average resources used to treat cases in all MS-DRGs. MS-DRG relative weights are recalculated every year to reflect changes in technology and medical practice in a budget neutral manner. Under the MS-DRG payment system, there can be significant delays in obtaining adequate reimbursement amounts for hospitals for new technologies such that reimbursement may be insufficient to permit broad acceptance by hospitals.
In addition to payments to hospitals for procedures using our technology, Medicare makes separate payments to physicians for their professional services. The American Medical Association, or AMA, has developed a coding system known as the Current Procedural Terminology, or CPT, codes, which have been adopted by the Medicare program to describe and develop payment amounts for certain physician services.
The Medicare physician fee schedule uses CPT codes (and other codes) as part of the determination of allowable payment amounts to physicians. In determining appropriate payment amounts for surgeons, CMS receives guidance from the AMA regarding the relative technical skill level, level of resources used, and complexity of a new surgical procedure. Generally, the designation of a new procedure code for a new procedure using a new product does not occur until after FDA clearance or approval of the product used in the procedure. Codes are assigned by either the AMA (for CPT codes) or CMS (for Medicare-specific codes) and new codes usually become effective on January 1st of each year.
85
One result of the current Medicare payment system, which is also utilized by most non-governmental third-party payors, is that a patient’s treating physician orders a particular service and the hospital (or other facility in which the procedure is performed) bears the cost of delivery of the service. Hospitals have limited ability to align their financial interests with that of the treating physician because Medicare law generally prohibits hospitals from paying physicians to assist in controlling the costs of hospital services, including paying physicians to limit or reduce services to Medicare beneficiaries even if such services are medically unnecessary. As a result, hospitals have traditionally stocked supplies and products requested by physicians and have had limited ability to restrict physician choice of products and services.
The Patient Protection and Affordable Care Act enacted on March 23, 2010, as amended by the Health Care and Education Reconciliation Act of 2010 enacted on March 30, 2010, or, together, the Health Care Reform Law, includes a number of provisions that will likely result in more coordination between hospitals and physicians resulting in the alignment of financial incentives between hospitals and physicians to control hospital costs. Most significantly, the Health Care Reform Law provides for the establishment of a Medicare shared savings program whereby Medicare will share certain savings realized in the delivery of services to Medicare beneficiaries with accountable care organizations, which may be organized through various different legal structures between hospitals and physicians. We expect that the overall result of such increased coordination will be voluntary reductions in the array of choices currently available to physicians with respect to diagnostic services, medical supplies and equipment. Such a reduction in physician choices may also result in hospitals reducing their overall number of vendors from which they purchase supplies, equipment and products. The Health Care Reform Law may make it more difficult for us to become and remain an approved vendor, which could have an adverse effect on our financial results and business.
Among other things, the Health Care Reform Law will ultimately increase the overall pool of persons with access to health insurance in the United States. Although such an increase in covered lives should ultimately benefit hospitals, the Health Care Reform Law, also includes a number of cuts in Medicare reimbursement to hospitals that may take effect prior to hospitals’ realizing the financial benefit of a larger pool of insured persons. Such cuts in Medicare reimbursement could adversely impact the operations and finances of hospitals reducing their ability to purchase medical devices such as our products. Further, the fact that the Health Care Reform Law did not address pending reductions of Medicare payments to physicians under the sustainable growth rate formula could result in an overall reduction of physicians willing to participate in Medicare.
Commercial Insurers
In addition to the Medicare program, many private payors look to CMS policies as a guideline in setting their coverage policies and payment amounts. The current coverage policies of these private payors may differ from the Medicare program, and the payment rates they make may be higher, lower, or the same as the Medicare program. If CMS or other agencies decrease or limit reimbursement payments for doctors and hospitals, this may affect coverage and reimbursement determinations by many private payors. Additionally, some private payors do not follow the Medicare guidelines, and those payors may reimburse only a portion of the costs associated with the use of our products, or none at all.
Fraud and Abuse Laws
Because of the significant federal funding involved in Medicare and Medicaid, Congress and the states have enacted, and actively enforce, a number of laws whose purpose is to eliminate fraud and abuse in federal healthcare programs. Our business is subject to compliance with these laws.
Anti-Kickback Laws
In the United States, there are federal and state anti-kickback laws that generally prohibit the payment or receipt of kickbacks, bribes or other remuneration in exchange for the referral of patients or other health-related business. The United States federal healthcare programs’ Anti-Kickback Statute makes it unlawful for individuals or entities knowingly and willfully to solicit, offer, receive or pay any kickback, bribe or other
86
remuneration, directly or indirectly, in exchange for or to induce the purchase, lease or order, or arranging for or recommending purchasing, leasing, or ordering, any good, facility, service, or item for which payment may be made in whole or in part under a federal healthcare program such as Medicare or Medicaid. The Anti-Kickback Statute covers “any remuneration,” which has been broadly interpreted to include anything of value, including for example gifts, certain discounts, the furnishing of free supplies, equipment or services, credit arrangements, payments of cash and waivers of payments. Several courts have interpreted the statute’s intent requirement to mean that if any one purpose of an arrangement involving remuneration is to induce referrals of federal healthcare covered business, the arrangement can be found to violate the statute. Penalties for violations include criminal penalties and civil sanctions such as fines, imprisonment and possible exclusion from Medicare, Medicaid and other federal healthcare programs. In addition, several courts have permitted kickback cases brought under the Federal False Claims Act to proceed, as discussed in more detail below.
Because the Anti-Kickback Statute is broadly written and encompasses many harmless or efficient arrangements, Congress authorized the Office of Inspector General of the United States Department of Health and Human Services, or OIG, to issue a series of regulations, known as “safe harbors.” For example, there are regulatory safe harbors for payments to bona fide employees, properly reported discounts, and payments for certain investment interests. Although an arrangement that fits into one or more of these exceptions or safe harbors is immune from prosecution, arrangements that do not fit squarely within an exception or safe harbor do not necessarily violate the statute. The failure of a transaction or arrangement to fit precisely within one or more of the exceptions or safe harbors does not necessarily mean that it is illegal or that prosecution will be pursued. However, conduct and business arrangements that arguably implicate the Anti-Kickback Statute but do not fully satisfy all the elements of an exception or safe harbor may be subject to increased scrutiny by government enforcement authorities such as the OIG. The Health Care Reform Law increases the investigatory authority of the OIG, clarifies that Anti-Kickback Statute claims can be brought under the federal civil False Claims Act, and provides for enhanced civil monetary penalties and expanded permissible exclusion authority.
Many states have laws that implicate anti-kickback restrictions similar to the Anti-Kickback Statute. Some of these state prohibitions apply regardless of whether federal healthcare program business is involved such as for self-pay or private pay patients.
Government officials have focused their enforcement efforts on marketing of healthcare services and products, among other activities, and recently have brought cases against companies, and certain sales, marketing and executive personnel, for allegedly offering unlawful inducements to potential or existing customers in an attempt to procure their business.
Federal Civil False Claims Act and State False Claims Laws
The federal civil False Claims Act imposes liability on any person or entity who, among other things, knowingly and willfully presents, or causes to be presented, a false or fraudulent claim for payment by a federal healthcare program, including Medicare and Medicaid. The “qui tam,” or “whistleblower,” provisions of the False Claims Act allow a private individual to bring actions on behalf of the federal government alleging that the defendant has submitted a false claim to the federal government, and to share in any monetary recovery. In recent years, the number of suits brought against healthcare providers by private individuals has increased dramatically. Medical device companies, like us, can be held liable under false claims laws, even if they do not submit claims to the government where they are deemed to have caused submission of false claims by, among other things, providing incorrect coding or billing advice about their products to customers that file claims, or by engaging in kickback arrangements with customers that file claims.
The False Claims Act also has been used to assert liability on the basis of misrepresentations with respect to the services rendered and in connection with alleged off-label promotion of products. Our future activities relating to the manner in which we sell our products and document our prices such as the reporting of discount and rebate information and other information affecting federal, state and third-party reimbursement of our products, and the sale and marketing of our products, may be subject to scrutiny under these laws.
87
The Health Care Reform Law is likely to increase the number of cases asserting civil False Claims Act violations since it removes a significant defense to such claims and clarifies that a violation of the Anti-Kickback Statute or retention of a federal healthcare program overpayment are actionable under the civil False Claims Act.
When an entity is determined to have violated the False Claims Act, it may be required to pay up to three times the actual damages sustained by the government, plus civil penalties of between $5,500 to $11,000 for each separate false claim. There are many potential bases for liability under the False Claims Act. A number of states have enacted false claim laws analogous to the federal civil False Claims Act and many of these state laws apply where a claim is submitted to any state or private third-party payor. In this environment, our engagement of physician consultants in product development and product training and education could subject us to similar scrutiny. We are unable to predict whether we would be subject to actions under the False Claims Act or a similar state law, or the impact of such actions. However, the costs of defending such claims, as well as any sanctions imposed, could significantly affect our financial performance.
HIPAA Fraud and Other Regulations
The Health Insurance Portability and Accountability Act of 1996, or HIPAA, created a class of federal crimes known as the “federal health care offenses,” including healthcare fraud and false statements relating to healthcare matters. The HIPAA healthcare fraud statute prohibits, among other things, knowingly and willfully executing, or attempting to execute, a scheme or artifice to defraud any healthcare benefit program, or to obtain by means of false of fraudulent pretenses, any money under the control of any healthcare benefit program, including private payors. A violation of this statute is a felony and may result in fines, imprisonment and/or exclusion from government-sponsored programs. The Health Care Reform Law also provides for civil monetary penalties for knowingly participating in certain federal healthcare offenses and enhances sentences under the Federal Sentencing Guidelines for such offenses. The HIPAA false statements statute prohibits, among other things, knowingly and willfully falsifying, concealing or covering up a material fact or making any materially false, fictitious or fraudulent statement or representation in connection with the delivery of or payment for healthcare benefits, items or services. A violation of this statute is a felony and may result in fines and/or imprisonment. Entities that are found to have aided or abetted in a violation of the HIPAA federal health care offenses are deemed by statute to have committed the offense and are punishable as a principal.
We are also subject to the United States Foreign Corrupt Practices Act and similar anti-bribery laws applicable in non-United States jurisdictions that generally prohibit companies and their intermediaries from making improper payments to non-United States government officials for the purpose of obtaining or retaining business. Because of the predominance of government sponsored healthcare systems around the world, most of our customer relationships outside of the United States will be with governmental entities and therefore subject to such anti-bribery laws.
HIPAA and Other Privacy Regulations
The regulations that implement HIPAA also establish uniform standards governing the conduct of certain electronic healthcare transactions and protecting the security and privacy of individually identifiable health information maintained or transmitted by healthcare providers, health plans and healthcare clearinghouses, which are referred to as “covered entities.” Several regulations have been promulgated under HIPAA’s regulations including: the Standards for Privacy of Individually Identifiable Health Information, or the Privacy Rule, which restricts the use and disclosure of certain individually identifiable health information, the Standards for Electronic Transactions, which establishes standards for common healthcare transactions, such as claims information, plan eligibility, payment information and the use of electronic signatures, and the Security Standards for the Protection of Electronic Protected Health Information, or the Security Rule, which requires covered entities to implement and maintain certain security measures to safeguard certain electronic health information. Although we do not believe we are a covered entity and therefore are not currently directly subject to these standards, we expect that our customers generally will be covered entities and may ask us to
88
contractually comply with certain aspects of these standards by entering into requisite business associate agreements. While the government intended this legislation to reduce administrative expenses and burdens for the healthcare industry, our compliance with certain provisions of these standards entails significant costs for us.
The Health Information Technology for Economic and Clinical Health Act, or the HITECH Act, which was enacted in February 2009, strengthens and expands the HIPAA Privacy and Security Rules and the restrictions on use and disclosure of patient identifiable health information. HITECH also fundamentally changed a business associate’s obligations by imposing a number of Privacy Rule requirements and a majority of Security Rule provisions directly on business associates that were previously only directly applicable to covered entities. HITECH includes, but is not limited to, prohibitions on exchanging patient identifiable health information for remuneration, restrictions on marketing to individuals and obligations to agree to provide individuals an accounting of virtually all disclosures of their health information. Moreover, HITECH requires covered entities to report any unauthorized use or disclosure of patient identifiable health information, known as a breach, to the affected individuals, the United States Department of Health and Human Services, or HHS, and depending on the size of any such breach, the media for the affected market. Business associates are similarly required to notify covered entities of a breach. Most of the HITECH provisions became effective in February 2010 and it is expected that the HHS will issue regulations to clarify many of the new provisions. HHS has already issued regulations governing breach notification which were effective in September 2009.
HITECH has increased civil penalty amounts for violations of HIPAA by either covered entities or business associates up to an annual maximum of $1.5 million for uncorrected violations based on willful neglect. Imposition of these penalties is more likely now because HITECH significantly strengthens enforcement. It requires HHS to conduct periodic audits to confirm compliance beginning in February 2010 and to investigate any violation that involves willful neglect which carries mandatory penalties beginning in February 2011. Additionally, state attorneys general are authorized to bring civil actions seeking either injunctions or damages in response to violations of HIPAA Privacy and Security Rules that threaten the privacy of state residents.
In addition to federal regulations issued under HIPAA, some states have enacted privacy and security statutes or regulations that, in some cases, are more stringent than those issued under HIPAA. In those cases, it may be necessary to modify our planned operations and procedures to comply with the more stringent state laws. If we fail to comply with applicable state laws and regulations, we could be subject to additional sanctions.
Federal and state consumer protection laws are being applied increasingly by the FTC and state attorneys general to regulate the collection, use, storage and disclosure of personal or patient information, through websites or otherwise, and to regulate the presentation of web site content. Courts may also adopt the standards for fair information practices promulgated by the FTC, which concern consumer notice, choice, security and access. Numerous other countries have or are developing laws governing the collection, use, disclosure and transmission of personal or patient information.
HIPAA as well as other federal and state laws apply to our receipt of patient identifiable health information in connection with research and clinical trials. We collaborate with other individuals and entities in conducting research and all involved parties must comply with applicable laws. Therefore, the compliance of the surgeons, hospitals or other providers or entities with whom we collaborate also impacts our business.
Employees
As of April 30, 2010, we had 23 full time employees, 11 of whom were engaged in research and development, six in manufacturing and clinical sales and six in general administrative and finance functions. None of our employees is covered by a collective bargaining agreement, and we consider our relationship with our employees to be good.
89
Facilities
We lease approximately 7,400 square feet of space in Irvine, California under a lease that expires in July 2012, which we use as our principal research and development facility and for the assembly of certain of our products. We have the right to extend our Irvine lease for three additional years upon prior written notice and the fulfillment of certain conditions.
We lease approximately 3,300 square feet of office space in Memphis, Tennessee, which we use as our executive offices. Our Memphis lease expires in November 2014. We also have a license to use approximately 1,400 square feet of space in Baltimore, Maryland, which we use as our advanced research and development facility. Our license agreement with respect to our Baltimore facility expires in February 2011.
We believe that our current facilities are sufficient to meet our needs for the foreseeable future.
Litigation
From time to time we may be involved in litigation relating to claims arising out of our operations.
On April 22, 2010, SurgiVision Consultants, Inc. and Guy M. Kezirian filed a lawsuit against us in the United States District Court, Central District of California, alleging trademark infringement, unfair competition, trademark dilution and violation of the Anti-Cybersquatting Protection Act, all relating to our use of our SURGI-VISION and SURGIVISION trademarks and our www.surgivision.com domain name. The plaintiffs are seeking unspecified monetary damages and injunctive relief. This action is at a very preliminary stage. We believe we have strong defenses to the allegations, and we intend to vigorously defend ourselves in the lawsuit to protect our own trademark rights.
90
Directors and Executive Officers
The following table sets forth information about our directors, executive officers and other key employees as of April 30, 2010.
| Name |
Age | Position(s) | ||
| Directors and Executive Officers |
||||
| Kimble L. Jenkins(4) |
48 | President, Chief Executive Officer and Chairman of Board of Directors | ||
| Lenox D. Baker(3) |
68 | Director | ||
| Paul A. Bottomley |
57 | Director | ||
| Charles E. Koob(2)(3)(4) |
65 | Director | ||
| James K. Malernee, Jr.(1)(3) |
62 | Director | ||
| Wendelin C. Maners |
47 | Director | ||
| Michael A. Pietrangelo(1)(2)(4) |
67 | Director | ||
| John N. Spencer, Jr.(1)(2)(4) |
69 | Director | ||
| John C. Thomas, Jr. |
56 | Director | ||
| Peter G. Piferi |
50 | Chief Operating Officer | ||
| David W. Carlson |
46 | Chief Financial Officer | ||
| Carol J. Barbre |
49 | Vice President, Product Management | ||
| John T. Keane |
44 | Vice President, Sales | ||
| Michael M. Moore |
37 | Vice President, Operations | ||
| Oscar L. Thomas |
39 | Vice President, Business Affairs and Secretary |
(1) Member of the Audit Committee
(2) Member of the Compensation Committee
(3) Member of the Corporate Governance and Nominating Committee
(4) Member of the Executive Committee
Kimble L. Jenkins joined our Board of Directors in September 2002 and presently serves as our Chairman. Mr. Jenkins has served as our President since January 2003, and he has also served as our Chief Executive Officer since September 2004. Mr. Jenkins served in those offices on a part-time basis until May 2008, at which time Mr. Jenkins began serving as our President and Chief Executive Officer on a full-time basis. Prior to May 2008, Mr. Jenkins was also a Managing Director with the investment bank Morgan Keegan & Company, Inc., where he founded that firm’s Private Equity Group in 1998. Mr. Jenkins has over 20 years of experience building and working with growth stage companies. Mr. Jenkins holds a Bachelor of Arts from Brown University and a Juris Doctorate from Georgetown University Law Center. As our Chief Executive Officer, Mr. Jenkins offers unique insight and vision into our operations, our competition and the medical device industry.
Lenox D. Baker joined our Board of Directors in December 1998. Pursuant to the terms of our First Amended and Restated Stockholders’ Agreement, as amended, or the Stockholders’ Agreement, which will terminate in connection with this offering, Dr. Baker is the designated nominee of Johns Hopkins to serve on our Board of Directors. He is Past-Chairman of the board of trustees for Johns Hopkins Medicine and Past Vice- Chairman of the board of trustees for Johns Hopkins. He currently serves on the executive committee and board of trustees of Johns Hopkins Medicine, as well as serving on the board of trustees of Johns Hopkins. Since 1979, Dr. Baker has practiced cardiothoracic surgery with Mid-Atlantic Cardiothoracic Surgeons and has served as its President since 2002. Since 1982, Dr. Baker has served as Chief of the Division of Cardiac and Thoracic Surgery at Sentara Norfolk General Hospital. In 1975, Dr. Baker founded Impra Inc., a manufacturer of prosthetic arterial grafts, and he served as its Chairman of the Board until Bard Cardiology acquired the company in 1996. Dr. Baker has served as the principal investigator in numerous studies and has written and published multiple articles in the field of cardiothoracic surgery. Dr. Baker also serves as a member of the board of directors of WellPoint, Inc., a publicly traded health benefits company. Dr. Baker offers a practicing physician’s perspective on the design, development and commercialization of our product candidates.
91
Paul A. Bottomley is a SurgiVision founder and has been a member of our Board of Directors since December 1998. Pursuant to the terms of the Stockholders’ Agreement, Dr. Bottomley is the designated nominee of the Scientific Founders, as such term is defined in the Stockholders’ Agreement, to serve on our Board of Directors. Dr. Bottomley joined Johns Hopkins in 1994. Since 1997, Dr. Bottomley has served as the Director of the Division of MR Research in the Department of Radiology at Johns Hopkins. Previously, Dr. Bottomley worked at General Electric Company’s Research and Development Center from 1980-1994 where he played a key role in the development of their MRI clinical product and was awarded the Center’s highest honor, its Coolidge Medal and Fellowship, for these developments in 1990. He was awarded the Society of Magnetic Resonance in Medicine’s Gold Medal for his contributions to MRI in 1989. He holds 34 U.S. patents and has written more than 150 scientific journal publications. Dr. Bottomley also serves as a consultant to us. As a pioneer in MR research, Dr. Bottomley offers expertise in the practical application of our technologies and the commercial opportunities for our product candidates.
Charles E. Koob joined our Board of Directors in August 2008. From 1970 to 2008, Mr. Koob practiced competition, trade regulation and antitrust law at the law firm of Simpson Thacher & Bartlett and served as the co-head of the firm’s litigation department for a portion of his tenure. For much of his career, Mr. Koob served as a strategic advisor for the boards of directors of many public companies. Mr. Koob also serves on the board of directors of MiMedx Group, Inc., a publicly traded biomedical products company. As a byproduct of Mr. Koob’s sophisticated former legal practice, Mr. Koob offers expertise in the areas of corporate governance, contract negotiation and organizational and strategic leadership.
James K. Malernee, Jr. joined our Board of Directors in March 2010. Dr. Malernee is a cofounder of Cornerstone Research, Inc., or Cornerstone Research, a consulting firm specializing in analytical support to attorneys in all phases of commercial litigation and regulatory proceedings, and he currently serves as Chairman and Managing Director of that firm. Over the last twenty years with Cornerstone Research, he has directed research on complex business issues related to a wide variety of cases. In recent years, Dr. Malernee has specialized in securities matters, supervising hundreds of cases dealing with material disclosure, loss causation, insider trading, mergers and acquisitions, targeted repurchases, minority buyouts, stock trading behavior, valuation and class certification. Dr. Malernee has served as a board member and consultant to major corporations, and he has taught finance at the University of Texas at Austin and business strategy at the Stanford Graduate School of Business. Through his academic and professional pursuits, Dr. Malernee offers expertise in finance and business strategy as well as an in-depth understanding of corporate disclosure and governance.
Wendelin C. Maners joined our Board of Directors in August 2008. Pursuant to the terms of the Stockholders’ Agreement, Ms. Maners is the designated nominee of Boston Scientific to serve on our Board of Directors. Ms. Maners has been employed by Boston Scientific since 1997 and currently is the Vice President, Strategy and Business Development. She is responsible for transaction and licensing activities for Boston Scientific’s neuromodulation, electrophysiology and undeveloped markets. Prior to joining Boston Scientific, Ms. Maners was Head of Healthcare Investment Banking at Barrington Associates, a merger & acquisition advisory firm in Los Angeles. With her background, Ms. Maners offers insight into the medical device industry, particularly as it relates to neurological applications and catheter-based cardiac ablation.
Michael A. Pietrangelo joined our Board of Directors in March 2010. From 1972 through 1989, Mr. Pietrangelo was employed by Schering-Plough Corporation in various capacities including President of the Personal Care Products Group. From 1989 to 1990, he served as President and Chief Operating Officer of Western Publishing Company. From 1990 to 1994, Mr. Pietrangelo was the President and Chief Executive Officer of CLEO, Inc., a subsidiary of Gibson Greetings, Inc. From 1994 until 1998, he served as President of Johnson Products Company, a subsidiary of IVAX Corporation. Since 1998, Mr. Pietrangelo has practiced law at Pietrangelo Cook PLC. Mr. Pietrangelo is also a director of Medicis Pharmaceutical Corporation, a publicly traded pharmaceutical company, serving on the executive committee (Chair), compliance committee (Chair), and nominating and governance committee. Mr. Pietrangelo also serves on the board of directors of the American Parkinson Disease Association, a not-for-profit organization focused on serving the Parkinson’s community, and Universal Insurance Holdings, Inc., a publicly traded insurance holding company. Mr. Pietrangelo currently
92
serves as the managing partner of Theraplex Company LLC, a privately held company. As a result of his diverse professional background, Mr. Pietrangelo offers a unique combination of legal expertise and operational acumen.
John N. Spencer, Jr. joined our Board of Directors in March 2010. Mr. Spencer is a certified public accountant and was a partner of Ernst & Young LLP where he spent more than 38 years until his retirement in 2000. Mr. Spencer serves on the board of directors of GeoVax Labs, Inc., a publicly traded biotechnology company, and until April 2009, served on the board of directors of Firstwave Technologies, Inc., formerly a publicly traded customer relationship management software company. In addition, he serves as a consultant to various companies, primarily relating to financial accounting and reporting matters. By virtue of his experience at Ernst & Young, where he was the partner in charge of its life sciences practice for the southeastern United States, which included a large number of publicly owned and privately held medical technology companies, together with his continuing expertise as a director of, and a consultant to, other publicly traded and privately held companies, Mr. Spencer offers expertise in accounting, finance and the medical device industry.
John C. Thomas, Jr. joined our Board of Directors in April 2004. From 1998 through April 23, 2010, Mr. Thomas served as our Chief Financial Officer on a part-time basis. Mr. Thomas also serves as a part-time chief financial officer and secretary for CorMatrix Cardiovascular, Inc. (2001 to present), a privately held medical device company, and Motion Reality, Inc. (2001 to present), a privately held motion capture and simulation company. Previously, Mr. Thomas served as a chief financial officer and secretary for the following companies: MiMedx Group, Inc. (2006 to 2009), a publicly traded biomedical products company; iVideotunes, Inc. (2005 to 2008) a privately held music company; and DARA Pharmaceuticals, Inc., or DARA (2002 to 2008). Mr. Thomas is a certified public accountant, and was formerly an auditor with Arthur Andersen & Company. There is no familial relationship between Mr. John C. Thomas, Jr. and Mr. Oscar L. Thomas. Mr. Thomas offers expertise in accounting, financial statement analysis, medical device company operations and the requirements of a growth stage company.
Peter G. Piferi joined us in December 2006 as our Chief Operating Officer. Mr. Piferi has 23 years of experience in the areas of product development, operations, engineering and production in the medical device industry. From March 2003 to December 2006, Mr. Piferi served as Vice President, Endovascular Technologies for Edwards Lifesciences Corporation. In addition, Mr. Piferi has served as Vice President at Kriton Medical Inc. and Orbus Medical Technologies, Inc. and as Director of Advanced Engineering at Cordis Corporation.
David W. Carlson joined us in February 2010 as Vice President, Finance and was promoted to Chief Financial Officer on April 23, 2010. Mr. Carlson has 17 years of experience in financial leadership roles in the medical device industry. From 1999 to 2009, he served in various financial management positions as a Vice President of Finance and Senior Finance Director at Medtronic, Inc., a global leader in medical technologies. He was serving as the Corporate Controller of Sofamor Danek, Inc., a then publicly traded medical device company, when it was acquired by Medtronic, Inc. in 1999. Mr. Carlson is a certified public accountant, and was formerly an auditor for PricewaterhouseCoopers LLP.
Carol J. Barbre joined us in May 2008 as Vice President, Product Management. Ms. Barbre has 20 years of experience in the medical device industry in the areas of marketing and business development, with a focus on new medical therapies. From May 2007 to May 2008, Ms. Barbre served as Senior Director of Marketing for Edwards Lifesciences Corporation, a publicly traded medical device company. From 2002 to May 2007, Ms. Barbre served as Global Marketing Director for Bolton Medical, Inc., a privately held medical device company.
John T. Keane joined us in April 2010 as Vice President, Sales. Mr. Keane has 20 years of sales experience in the medical device industry. From October 2006 until April 2010, Mr. Keane served as the Worldwide Director of Sales for Stereotactic Surgery, Radiosurgery, Image Guided Surgery, Brain Mapping and Service Agreements for Integra Radionics, Inc., a subsidiary of Integra Lifesciences Corporation, a publicly traded medical device manufacturer. From 2004 to 2006, Mr. Keane served as an Academic Center Representative for I-Flow Corporation, formerly a publicly traded medical device company that merged with a subsidiary of
93
Kimberly-Clark Corporation, a publicly traded corporation, in 2009. From 1996 to 2004, Mr. Keane was the National Leader of Academic Sales Representatives at Baxter International Inc., a publicly traded global, diversified health care company.
Michael M. Moore joined us in October 2008 as Senior Director, Operations, and he was promoted to Vice President, Operations in June 2009. Mr. Moore has 18 years of experience in medical device development and product realization. From January 2003 to March 2008, he was the Chief Technical Officer for Bolton Medical, Inc. In addition, Mr. Moore previously served as Director of R&D and Operations for AVE-Peripheral Vascular, a division of Medtronic, Inc., and in different operations and product development roles at Cordis Corporation and DePuy Orthopedics, Inc.
Oscar L. Thomas joined us in April 2008 as Vice President, Business Affairs. In addition, Mr. Thomas serves as our Secretary. From January 2003 to April 2008, Mr. Thomas was a partner in the Corporate and Securities Practice Group of the law firm Bass, Berry & Sims PLC. There is no familial relationship between Mr. John C. Thomas, Jr. and Mr. Oscar L. Thomas.
Board Composition
Upon the completion of this offering, we will have a Board of Directors consisting of nine members. In accordance with the terms of our certificate of incorporation and our bylaws, which will become effective upon completion of this offering, the Board of Directors will be divided into three classes, Class I, Class II and Class III, with each class serving staggered three-year terms. Upon the completion of this offering, the members of the classes will be divided as follows:
| • | the Class I directors will be Mr. Jenkins, Mr. Pietrangelo and Ms. Maners, and their term will expire at the annual meeting of stockholders to be held in 2011; |
| • | the Class II directors will be Mr. Thomas, Dr. Malernee and Dr. Baker, and their term will expire at the annual meeting of stockholders to be held in 2012; and |
| • | the Class III directors will be Mr. Spencer, Mr. Koob and Dr. Bottomley, and their term will expire at the annual meeting of stockholders to be held in 2013. |
Our certificate of incorporation that will become effective upon the completion of this offering provides that the authorized number of directors may be changed only by resolution of the Board of Directors. Any additional directorships resulting from an increase in the number of directors will be distributed between the three classes so that, as nearly as possible, each class will consist of one-third of the directors. This classification of the Board of Directors may have the effect of delaying or preventing changes in our control or management.
Our directors may be removed only for cause by the affirmative vote of the holders of a majority of our voting stock.
Board Committees and Independence
Rule 5605 of the Nasdaq Marketplace Rules requires a majority of a listed company’s board of directors to be comprised of independent directors within one year of listing. In addition, Nasdaq Marketplace Rules require that, subject to specified exceptions, each member of a listed company’s audit, compensation and nominating and governance committees be independent and that audit committee members also satisfy independence criteria set forth in Rule 10A-3 under the Securities Exchange Act of 1934, as amended, or the Exchange Act. Under Rule 5605(a)(2), a director will only qualify as an “independent director” if, in the opinion of our Board of Directors, that person does not have a relationship that would interfere with the exercise of independent judgment in carrying out the responsibilities of a director. In order to be considered independent for purposes of Rule 10A-3, a member of an audit committee of a listed company may not, other than in his or her capacity as a member of the audit committee, the Board of Directors, or any other board committee: (1) accept, directly or indirectly, any consulting, advisory, or other compensatory fee from the listed company or any of its subsidiaries; or (2) be an affiliated person of the listed company or any of its subsidiaries.
94
Our Board of Directors undertook a review of the composition of our Board of Directors and its committees and the independence of each director. Based upon information requested from and provided by each director concerning his or her background, employment and affiliations, including family relationships, our Board of Directors has determined that none of Drs. Baker, Bottomley or Malernee, Messrs. Koob, Spencer or Pietrangelo or Ms. Maners, representing seven of our nine directors, has a relationship that would interfere with the exercise of independent judgment in carrying out the responsibilities of a director and that each of these directors is “independent” as that term is defined under Rule 5605(a)(2) of the Nasdaq Marketplace Rules. In making such determination, our Board of Directors considered the relationships that each such director has with us and all other facts and circumstances the Board of Directors deemed relevant in determining independence, including the beneficial ownership of our capital stock by each director.
Board Committees
Our Board of Directors has an audit committee, a compensation committee, a corporate governance and nominating committee and an executive committee.
Audit Committee
Our audit committee consists of Messrs. Spencer (Chair) and Pietrangelo and Dr. Malernee. The functions of the audit committee include:
| • | appointing, determining the compensation of and overseeing the work of the independent registered public accounting firm; |
| • | pre-approving all auditing services and permitted non-audit services, including the fees and terms thereof, to be performed by the independent registered public accounting firm; |
| • | reviewing and discussing with management and the independent registered public accounting firm the annual audited and quarterly unaudited financial statements and our disclosure under “Management’s Discussion and Analysis of Financial Condition and Results of Operations” in our 10-Qs and 10-Ks; |
| • | reviewing and discussing the adequacy and effectiveness of our systems of internal accounting and financial controls; |
| • | overseeing our risk assessment and risk management relative to our financial risk exposure; |
| • | establishing procedures for the receipt, retention and treatment of complaints regarding accounting, internal accounting controls or auditing matters and the confidential, anonymous submission by employees of concerns regarding questionable accounting or auditing matters; and |
| • | issuing the audit committee report for inclusion in our proxy statement for our annual meeting. |
Our Board of Directors has determined that at this time, Mr. Spencer is an audit committee financial expert within the meaning of SEC regulations and the Nasdaq listing standards. Our Board of Directors has determined that each member of the audit committee satisfies the independence requirements for service on the audit committee. Both our independent registered public accounting firm and management will periodically meet privately with our audit committee.
Upon the effectiveness of the registration statement of which this prospectus forms a part, a copy of the charter for our audit committee will be posted on our website at www.surgivision.com. The inclusion of our website address in this prospectus does not include or incorporate by reference the information on our website into this prospectus.
Compensation Committee
Our compensation committee consists of Messrs. Pietrangelo (Chair), Koob and Spencer. The functions of the compensation committee include:
| • | determining the compensation of our executive officers and reviewing and approving the compensation goals and objectives relevant to such compensation; |
95
| • | reviewing and approving the compensation programs, plans and awards for our executive officers; |
| • | overseeing our risk assessment and risk management relative to our compensation structure and benefits plan administration; |
| • | administering and implementing our incentive compensation plans and equity-based plans; |
| • | reviewing and discussing with management the information contained in the Compensation Discussion and Analysis section of our proxy statement; and |
| • | issuing the compensation committee report on executive compensation for inclusion in our proxy statement for our annual meeting. |
Each member of our compensation committee is a non-employee director, as defined in Rule 16b-3 promulgated under the Exchange Act, and an outside director, as defined pursuant to Section 162(m) of the Code. Furthermore, our Board of Directors has determined that each member of our compensation committee satisfies the independence standards for compensation committees established by the Nasdaq Marketplace Rules.
Upon the effectiveness of the registration statement of which this prospectus forms a part, a copy of the charter for our compensation committee will be posted on our website at www.surgivision.com. The inclusion of our website address in this prospectus does not include or incorporate by reference the information on our website into this prospectus.
Corporate Governance and Nominating Committee
Our corporate governance and nominating committee consists of Mr. Koob (Chair) and Drs. Baker and Malernee. The functions of the corporate governance and nominating committee include:
| • | evaluating director performance on the Board of Directors and applicable committees of the Board of Directors; |
| • | interviewing, evaluating, nominating and recommending individuals for membership on our Board of Directors; |
| • | evaluating nominations by stockholders of candidates for election to our Board of Directors; |
| • | reviewing and recommending to our Board of Directors any amendments to our corporate governance documents; and |
| • | making recommendations to the Board of Directors regarding management succession planning. |
Our Board of Directors has determined that each member of the corporate governance and nominating committee satisfies the independence standards for the corporate governance and nominating committees established by the Nasdaq Marketplace Rules.
Upon the effectiveness of the registration statement of which this prospectus forms a part, a copy of the charter for our corporate governance and nominating committee will be posted on our website at www.surgivision.com. The inclusion of our website address in this prospectus does not include or incorporate by reference the information on our website into this prospectus.
Executive Committee
Our executive committee consists of Messrs. Jenkins (Chair), Koob, Pietrangelo and Spencer. The executive committee, which acts on behalf of the Board of Directors between regular meetings of the Board of Directors or at such times as our business so requires, has and may exercise all of the Board of Directors’ powers and authority in the management of our business and affairs, but the executive committee does not have the power or authority with respect to the following matters: (1) approving or adopting, or recommending to the stockholders, any action or matter expressly required by the Delaware General Corporation Law to be submitted to stockholders for approval; or (2) adopting, amending or repealing our bylaws.
96
Upon the effectiveness of the registration statement of which this prospectus forms a part, a copy of the charter for our executive committee will be posted on our website at www.surgivision.com. The inclusion of our website address in this prospectus does not include or incorporate by reference the information on our website into this prospectus.
Code of Business Conduct and Ethics
Our Board of Directors has adopted a Code of Business Conduct and Ethics, to be effective upon completion of this offering. The Code of Business Conduct and Ethics will apply to all of our employees, officers (including our principal executive officer, principal financial officer, principal accounting officer or controller, or persons performing similar functions), agents and representatives, including directors and consultants. Upon the effectiveness of the registration statement of which this prospectus forms a part, the full text of our Code of Business Conduct and Ethics will be posted on our website at www.surgivision.com. We intend to disclose future amendments to certain provisions of our Code of Business Conduct and Ethics, or waivers of such provisions, applicable to any principal executive officer, principal financial officer, principal accounting officer or controller, persons performing similar functions or our directors on our website identified above. The inclusion of our website address in this prospectus does not include or incorporate by reference the information on our website into this prospectus.
Compensation Committee Interlocks and Insider Participation
No member of our compensation committee has ever been an executive officer or employee of ours. None of our executive officers currently serves, or has served during the last completed fiscal year, on the compensation committee or board of directors of any other entity that has one or more executive officers serving as a member of our Board of Directors or compensation committee.
Compensation Risks
We have assessed our compensation programs and have concluded that our compensation policies and practices do not create risks that are reasonably likely to have a material adverse effect on the company. Our compensation program is relatively simple and has only three material elements: base salary; annual bonus; and long-term equity compensation. Base salary represents a fixed amount of payment and therefore does not encourage any excessive risk taking. The compensation committee has determined annual bonus amounts by subjectively analyzing company and individual performance for the prior year and only rewarding individual and company performance that, in the opinion of the compensation committee, had a positive effect on stockholder value. The subjective nature of the compensation committee’s determinations regarding both the award and the amount of annual bonuses and equity grants provides a significant control over the incentive of an employee to take undue risk in order to receive a larger annual bonus or equity grant. Finally, our long-term equity compensation program involves only the issuance of options to our employees. We believe that the equity component of our compensation program serves to align the interest of management with the interests of stockholders and does not encourage excessive risk taking. Based on the foregoing, we believe that our compensation policies and practices do not create inappropriate or unintended significant risk to the company as a whole. We also believe that our compensation arrangements provide incentives that do not encourage risk-taking beyond the organization’s ability to effectively identify and manage significant risks; are compatible with effective internal controls and the risk management practices of the company; and are supported by the oversight and administration of the compensation committee with regard to executive compensation programs.
Director Compensation
Non-Employee Director Compensation Practices
In June 2010, we adopted the following compensation practices for our non-employee directors, which will become effective upon completion of this offering.
97
Retainers, Fees and Expenses
The following table sets forth the cash compensation to be paid to our non-employee directors:
| Board of Directors: |
|||
| Annual retainer per director |
$ | 35,000 | |
| Fee per meeting for a full board meeting |
$ | 0 | |
| Audit Committee: |
|||
| Annual retainer for chairperson |
$ | 12,000 | |
| Annual retainer for other members |
$ | 5,000 | |
| Fee per meeting |
$ | 0 | |
| Compensation Committee: |
|||
| Annual retainer for chairperson |
$ | 8,000 | |
| Annual retainer for other members |
$ | 5,000 | |
| Fee per meeting |
$ | 0 | |
| Corporate Governance and Nominating Committee: |
|||
| Annual retainer for chairperson |
$ | 8,000 | |
| Annual retainer for other members |
$ | 5,000 | |
| Fee per meeting |
$ | 0 |
The above retainers will be paid in quarterly installments. We also reimburse each non-employee director for reasonable travel and other expenses in connection with attending meetings of the Board of Directors.
Stock Options—Initial Grant
Upon an individual first becoming a non-employee director, the new director will receive a stock option grant equaling approximately $100,000 in value (based on our use of the Black-Scholes valuation model), rounded up to the nearest whole share; provided, however, that the number of shares underlying the grant will not exceed 18,750 shares, subject to adjustment to reflect and take into account any unusual or non-recurring transaction that affects our common stock, including a recapitalization, stock split, reverse stock split, split-up, combination or other similar corporate transaction or event. Such options will vest in equal annual installments over three years. The exercise price of these options will equal the closing price of our common stock on the date of grant.
Stock Options—Annual Grants
Any individual who serves as a non-employee director on the day following an annual meeting of our stockholders will receive a stock option grant. The number of shares underlying these annual grants will equal approximately $50,000 in value (based on our use of the Black-Scholes valuation model), rounded up to the nearest whole share; provided, however, that the number of shares underlying the grant will not exceed 7,500 shares, subject to adjustment to reflect and take into account any unusual or non-recurring transaction that affects our common stock, including a recapitalization, stock split, reverse stock split, split-up, combination or other similar corporate transaction or event. Such options will vest on the earlier of the first anniversary of the grant date or the day immediately preceding the next annual meeting of stockholders. The exercise price of these options will equal the closing price of our common stock on the date of grant.
Term of Stock Options
Each non-employee director stock option will terminate upon the earlier to occur of: (i) ten years from the date of grant; (ii) 12 months after the director dies; (iii) 12 months after the director ceases to be a director due to disability; or (iv) three months after the director ceases to be a director for any reason other than death or disability.
98
Stock Options—2010 Grants
In connection with this offering, we are granting stock options to each of our non-employee directors, the exercise price of which will be the initial public offering price. Each non-employee director will receive two awards:
| • | a stock option equaling approximately $100,000 in value (based on our use of the Black-Scholes valuation model), but not to exceed 18,750 shares, which option will vest in equal annual installments over three years; and |
| • | a stock option equaling approximately $50,000 in value (based on our use of the Black-Scholes valuation model), but not to exceed 7,500 shares, which option will vest on the earlier of the first anniversary of the grant date or the day immediately preceding our 2011 annual meeting of stockholders. |
2010 Incentive Compensation Plan
All of our directors are eligible to participate in our 2010 Incentive Compensation Plan and awards will be granted pursuant to the terms of that plan, as more fully described in the section entitled “Benefit Plans—2010 Incentive Compensation Plan”.
2009 Non-Employee Director Compensation
The following table sets forth information with respect to the compensation of all our non-employee directors in 2009, which was paid prior to the adoption of the revised compensation practices described above.
| Name |
Fees Earned or Paid in Cash ($) |
Option Awards ($)(1) |
All Other Compensation ($) |
Total ($) | ||||||||||
| Lenox D. Baker |
$ | 10,750 | $ | 8,100 | — | $ | 18,850 | |||||||
| Paul A. Bottomley |
10,500 | 8,100 | $ | 60,000 | (2) | 78,600 | ||||||||
| Charles E. Koob |
14,250 | 8,100 | — | 22,350 | ||||||||||
| Wendelin Maners(3) |
10,000 | 8,100 | — | 18,100 | ||||||||||
| Parker H. Petit(4) |
14,750 | — | — | 14,750 | ||||||||||
| (1) | Amounts represent the aggregate grant date fair value of such options as computed in accordance with ASC Topic 718 “Compensation—Stock Compensation,” or ASC Topic 718. For a discussion of the assumptions made in the valuation of these awards, see note 2 to the financial statements included elsewhere in this prospectus and the discussion under “Management’s Discussion and Analysis of Financial Condition and Results of Operations—Critical Accounting Policies and Significant Judgements and Estimates—Share-based compensation”. |
| (2) | This amount was compensation paid under Dr. Bottomley’s consulting agreement. |
| (3) | Ms. Maners remits all fees received in connection with her service as a director to Boston Scientific, who designated her as a nominee to serve on our Board of Directors pursuant to the Stockholders’ Agreement. In addition, Ms. Maners holds her options for the benefit of Boston Scientific. |
| (4) | On December 22, 2009, Mr. Petit resigned from our Board of Directors. Like the other directors, Mr. Petit received an option to purchase 2,500 shares of our common stock on December 10, 2009; however, no award agreement was issued to Mr. Petit and the shares were forfeited upon Mr. Petit’s resignation on December 22, 2009. Mr. Petit’s resignation was not the result of any disagreement with us on any matter relating to our operations, policies or practices. Mr. Petit had recently become the Chairman of the Board, Chief Executive Officer and President of MiMedx Group, Inc., a publicly traded biomedical products company. Mr. Petit advised us that, given the scope of his responsibilities at MiMedx, he did not believe that he would be able to devote sufficient time to serve on our Board of Directors. |
Compensation Discussion and Analysis
Introduction
Our compensation discussion and analysis discusses the total compensation for our named executive officers, and it describes our overall compensation philosophy, objectives and practices. Our compensation philosophy and objectives generally apply to all of our employees and all of our employees are eligible to participate in the main components of our compensation program: salary; annual bonus; and equity compensation. The relative value of each of these components for individual employees varies based on job role and responsibility, as well as our financial performance.
99
Compensation Philosophy and Objectives
Our compensation approach is necessarily tied to our stage of development. Our compensation philosophy is to offer our executive officers, including our named executive officers, compensation and benefits that meet our goals of attracting, retaining and motivating highly skilled management, which is necessary to achieve our financial and strategic objectives and create long-term value for our stockholders. Accordingly, our executive officer compensation program is designed to link compensation to corporate and individual performance and to align executive officers’ interests with stockholder value creation by subjectively analyzing both corporate and individual performance in determining appropriate base salary, bonus and equity compensation awards.
We believe compensation should be determined within a framework that is intended to reward individual contribution and the achievement of company objectives. Within this overall philosophy, our objectives are to:
| • | attract, retain and motivate our executives by providing a total compensation program that takes into consideration our strategic business needs; |
| • | align the financial interests of the executive officers with those of our stockholders, both in the short and long term; |
| • | provide incentives for achieving and exceeding performance expectations; and |
| • | appropriately reward executive officers for creating long-term stockholder value. |
Prior to June 2010, each of our named executive officers was an “at-will” employee; however, some of our named executive officers had employment letters that set forth the basic terms of their employment. In connection with this offering, in June 2010, we entered into employment agreements with each of our named executive officers other than Mr. John C. Thomas, Jr.
On an annual basis, our compensation committee has utilized its business judgment to establish:
| • | base salaries for our named executive officers based on the recommendations of our Chief Executive Officer and the compensation committee’s exercise of its subjective judgment; |
| • | annual cash bonuses based on the recommendations of our Chief Executive Officer and a subjective analysis by the compensation committee of both our performance and each named executive officer’s performance for the most recently completed fiscal year; and |
| • | any long term equity compensation awards to the named executive officers based on the recommendations of the Chief Executive Officer and the compensation committee’s exercise of its subjective judgment. |
Role of Directors and Executive Officers in Setting Compensation
Prior to September 2008, we did not have a compensation committee and compensation decisions for our named executive officers were approved by our Board of Directors upon the recommendation of our Chief Executive Officer. The compensation recommendations of our Chief Executive Officer have been largely discretionary, based on our Chief Executive Officer’s subjective assessment of the particular executive officer, publicly available data relating to compensation of executive officers at other medical device companies and input from our other executive officers. There is no particular mathematical formula for deriving executive compensation from these sources. As we gain experience as a public company, we expect that the specific direction, emphasis and components of our executive compensation program will continue to evolve. For example, over time, we expect to reduce our reliance upon subjective determinations made by our Chief Executive Officer in favor of a more empirically-based approach, that could involve benchmarking the compensation paid to our named executive officers against peer companies that we identify and the use of clearly defined, objective targets to determine incentive compensation awards.
The compensation committee typically considers, but is not required to accept, the recommendations of our Chief Executive Officer regarding the performance and proposed base salary and bonus and equity awards for the
100
other named executive officers, as well as himself. The compensation committee may also request the assistance of our Chief Financial Officer in evaluating the financial, accounting and tax implications of various compensation awards paid to the named executive officers. However, our Chief Financial Officer does not recommend or determine the amounts or types of compensation paid to the named executive officers. Our Chief Executive Officer and certain of our other named executive officers may attend compensation committee meetings, as requested by the compensation committee. None of our named executive officers, including our Chief Executive Officer, attend any portion of the compensation committee meetings during which his or her compensation is established and approved.
We believe that the levels of compensation we provide should be appropriate for our business needs and circumstances. To date, the compensation committee has not engaged a compensation consultant. Rather, the compensation committee and our Chief Executive Officer applied subjective discretion to make compensation decisions and they have not used a specific formula or matrix to set compensation in relation to compensation paid by other medical device companies. Our compensation committee designed our executive compensation program based on the compensation committee’s general knowledge of compensation practices and the application of such knowledge to successfully attract and retain the named executive officers. Our compensation committee has not established any percentile targets for the levels of compensation provided to our named executive officers. To date, the compensation committee has not performed reviews of our compensation programs with those of similarly-situated companies, nor has it engaged in benchmarking of compensation paid to our named executive officers. Our historical approach has been to consider compensation practices and relevant factors rather than establishing compensation at specific benchmark percentiles. This enabled us to respond to dynamics in the labor market and provided us with flexibility in maintaining and enhancing our named executive officers’ engagement, focus, motivation and enthusiasm for our future. However, as mentioned above, we expect to build some of these objective practices into our compensation approach over time.
The amount of past compensation, including annual discretionary bonus awards, and amounts realizable from prior stock option awards, is generally not a significant factor in the compensation committee’s considerations, because these awards would have been earned based on prior years’ performances or granted in connection with a named executive officer’s initial hire.
Our named executive officers are not subject to mandated stock ownership or stock retention guidelines. It is the belief of the compensation committee that the equity component of our executive compensation program ensures that our named executive officers are also owners and those components work to align the named executive officers’ goals with the best interests of stockholders.
Elements of Our Executive Compensation Program
The principal elements of our executive compensation program have been base salary, a discretionary cash bonus and long-term equity compensation in the form of stock options. Each of these compensation elements satisfies one or more of our compensation objectives.
We have not adopted any policies with respect to long-term versus currently-paid compensation, but feel that both elements are necessary for achieving our compensation objectives. Currently-paid compensation provides financial stability for each of our named executive officers and immediate reward for short-term company and individual performance, while long-term compensation rewards achievement of strategic long-term objectives and contributes toward overall stockholder value. Similarly, while we have not adopted any policies with respect to cash versus equity compensation, we feel that it is important to encourage or provide for a meaningful amount of equity ownership by our named executive officers as to help align their interests with those of stockholders, one of our compensation objectives. We combine the compensation elements for each named executive officer in a manner that the compensation committee believes, in its discretion and judgment, is consistent with the executive’s contributions to our company and our overall goals with respect to executive compensation.
101
Base Salary
We believe that base salary is an important component of compensation as it provides a degree of financial stability for our named executive officers and is critical to recruiting and retaining our named executive officers. Base salary is also designed to recognize the scope of responsibilities placed on each named executive officer and reward each executive for his or her unique leadership skills, management experience and contributions. We make a subjective determination of base salary after considering such factors collectively.
Annual Cash Bonuses
Our cash bonus compensation is designed to motivate executives to achieve superior performance in their areas of responsibility. To date, we have awarded only discretionary annual cash bonuses based upon a subjective evaluation of corporate and individual performance by the compensation committee or, prior to its creation, our Board of Directors.
Long-Term Equity Compensation
We grant stock options to our named executive officers, as we believe that such grants further our compensation objectives of aligning the interests of our named executive officers with those of our stockholders, encouraging long-term performance, and providing a simple and easy-to-understand form of equity compensation that promotes executive retention. We view such grants both as incentives for future performance and as compensation for past accomplishments.
We generally have used stock options, rather than other forms of long-term incentives, because they create value for the executive only if stockholder value is increased through an increased share price. Prior to this offering, all stock option grants were made pursuant to either our 1998 Stock Option Plan or our 2007 Stock Incentive Plan. Our Board of Directors determined the exercise price based on internal or third-party valuation reports. In connection with and following this offering, all option grants will be made pursuant to our 2010 Incentive Compensation Plan. The exercise price of stock options will be the fair market value of our common stock on the grant date.
In lieu of receiving an initial stock option grant upon initial hire, our Chief Executive Officer purchased 500,000 shares of our common stock in September 2004. Mr. John C. Thomas, Jr., who was our Chief Financial Officer from 1998 until April 23, 2010, did not receive an equity grant upon initial hire in 1998. Our other named executive officers received equity grants in connection with their initial hire. The number of stock options granted to our named executive officers in connection with their initial hire was determined based on negotiations with each executive, represented the number necessary to recruit each executive from their then-existing positions and reflected our Board of Directors’ subjective evaluation of the executive’s experience and potential for future performance. We have made discretionary grants of equity compensation, from time to time, as determined by the Board of Directors or after its creation, the compensation committee, taking into consideration such factors as individual performance and market conditions. The timing of any such equity grants was determined by the Board of Directors’ determination of achievement by the named executive officer, and not any effort to time the grants in coordination with changes in our stock price.
Stock Ownership Guidelines
We currently do not have stock ownership guidelines.
Perquisites and Other Benefits
As a general matter, we do not intend to offer perquisites or other benefits to any executive officer, including the named executive officers, with an aggregate value in excess of $10,000, because we believe we can provide better incentives for desired performance with compensation in the forms described above. We recognize that, from time to time, it may be appropriate to provide some perquisites or other benefits in order to attract, motivate and retain our executives, with any such decision to be reviewed and approved by the compensation committee as needed.
102
Our named executive officers are eligible to participate in standard employee benefit plans, including medical, dental, vision, life and any other employee benefit or insurance plan made available to employees. We maintain a 401(k) plan, which is intended to be a tax-qualified defined contribution plan under Section 401(k) of the Internal Revenue Code, or the Code. In general, all of our U.S. employees are eligible to participate in this plan. The 401(k) plan includes a salary deferral arrangement pursuant to which participants may elect to reduce their current compensation by up to 90% or the statutory limit, $16,500 in 2009, whichever is less, and have the amount of the reduction contributed to the 401(k) plan. We made no matching contributions during 2009; however, we may add this benefit in the future for all employees.
Analysis of 2009 Compensation for Named Executive Officers
Base Salary
The base salary of Mr. Kimble L. Jenkins, our President and Chief Executive Officer, remained unchanged at $325,000 per year.
The base salary of Mr. John C. Thomas, Jr., our Chief Financial Officer in 2009, was increased from $60,000 per year to $100,000 per year to reflect Mr. Thomas’ agreement to devote additional time to our affairs. Mr. Thomas was a part-time employee and he ceased serving as our Chief Financial Officer on April 23, 2010.
The base salary of Mr. Peter G. Piferi, our Chief Operating Officer, remained unchanged in 2009 at $250,000 per year.
The base salary of Mr. Oscar L. Thomas, our Vice President, Business Affairs, remained unchanged in 2009 at $175,000 per year. Mr. Thomas is also entitled to receive guaranteed bonus payments equal to $12,500 per calendar quarter in accordance with the initial terms of his hiring.
The base salary of Mr. Michael M. Moore, our Vice President, Operations, was increased from $165,000 per year to $175,000 per year in 2009 to reflect the additional roles and responsibilities of Mr. Moore resulting from his promotion from Senior Director, Operations to Vice President, Operations.
Annual Cash Bonuses
In January 2010, our compensation committee authorized the payment of a discretionary annual bonus to each of our named executive officers as follows:
| Named Executive Officer |
Discretionary Bonus | ||
| Kimble L. Jenkins |
$ | 110,000 | |
| John C. Thomas, Jr. |
$ | 40,000 | |
| Peter G. Piferi |
$ | 100,000 | |
| Oscar L. Thomas |
$ | 80,000 | |
| Michael M. Moore |
$ | 35,000 | |
The bonuses were based upon recommendations made to the compensation committee by Mr. Jenkins. Mr. Jenkins described the performance of Messrs. John Thomas, Piferi, Oscar Thomas and Moore to the compensation committee and made a recommendation with respect to their annual bonus amounts, as well as his own. The bonus recommendations for Messrs. Jenkins, John Thomas and Oscar Thomas took into account the consummation of the co-development agreement with Siemens, the closing of the convertible note financing with Boston Scientific, and the filing of a registration statement for this offering. In addition, Mr. Jenkins’ bonus recommendation took into account the progress in obtaining 510(k) marketing clearance from the FDA for our ClearPoint system. The bonus recommendations for Messrs. Piferi and Moore took into account completion of development of our ClearPoint system, the successful design and implementation of our quality management system, and the progress in obtaining 510(k) marketing clearance from the FDA for our ClearPoint system.
103
The compensation committee then discussed in executive session Mr. Jenkins’ recommendations for the named executive officers, including an annual bonus for Mr. Jenkins. After subjectively evaluating both the performance of the company and the individuals under consideration, the compensation committee awarded to our named executive officers the annual cash bonuses indicated above.
Long-Term Equity Compensation
None of the named executive officers received long-term equity compensation in 2009 for their service as employees. Messrs. Jenkins and John Thomas received an option grant relating to their service as members of the Board of Directors that was identical to grants awarded to the non-employee directors in accordance with our then existing director compensation policy. In addition, Mr. Jenkins received an option grant in connection with a stock purchase transaction with us in December 2009.
Effect of Accounting and Tax Treatment on Compensation Decisions
In the review and establishment of our compensation programs, we consider the anticipated accounting and tax implications to us and our executives. While we consider the applicable accounting and tax treatment, these factors alone are not dispositive, and we also consider the cash and non-cash impact of the programs and whether a program is consistent with our overall compensation philosophy and objectives.
Section 162(m) of the Code imposes a limit on the amount of compensation that we may deduct in any one year with respect to covered employees, unless specific and detailed criteria are satisfied. Performance-based compensation, as defined in the Code, is fully deductible if the programs are approved by stockholders and meet other requirements. In general, we have determined that we will not seek to limit executive compensation so that all of such compensation is deductible under Section 162(m). However, from time to time, we monitor whether it might be in our interests to structure our compensation programs to satisfy the requirements of Section 162(m). We seek to maintain flexibility in compensating our executives in a manner designed to promote our corporate goals and, as a result, our compensation committee has not adopted a policy requiring all compensation to be deductible. Our compensation committee will continue to assess the impact of Section 162(m) on our compensation practices and determine what further action, if any, is appropriate.
Conclusion
The compensation committee believes that our executive leadership is a key element to our success and that the compensation package offered to our named executive officers is a key element in attracting and retaining the appropriate personnel.
The Board of Directors and, since its creation, the compensation committee each believes it has maintained compensation for our named executive officers at levels that are reflective of the talent and success of the individuals being compensated, and with the inclusion of additional compensation directly tied to performance, the compensation committee believes executive compensation will be sufficiently comparable to our industry peers to allow us to retain our key personnel at costs which are appropriate for us.
The compensation committee will continue to develop, analyze and review its methods for aligning executive officers’ long-term compensation with the benefits generated for stockholders. The compensation committee believes the idea of creating ownership helps align management’s interests with the interests of stockholders. The compensation committee has no pre-determined timeline for implementing new or ongoing long-term incentive plans. New plans are reviewed, discussed and implemented as the compensation committee feels it is necessary or appropriate as a measure to incent, retain and reward our named executive officers.
104
Summary Compensation Table
The following table shows the compensation awarded or paid to, or earned by, our Chief Executive Officer, Chief Financial Officer and our three other most highly compensated executive officers for the years ended December 31, 2009 and 2008. We refer to these executive officers in this prospectus as our “named executive officers”.
| Name and Principal Position |
Year | Salary ($) |
Bonus ($) |
Option Awards ($)(1) |
All Other Compensation ($)(2) |
Total ($) |
|||||||||||||||
| Kimble L. Jenkins |
2009 | $ | 325,000 | $ | 110,000 | $ | 192,060 | (3)(4) | $ | 5,355 | $ | 657,415 | (4) | ||||||||
| Chief Executive Officer and President |
2008 | 291,667 | 75,000 | 12,900 | 3,005 | 387,572 | |||||||||||||||
| John C. Thomas, Jr. |
2009 | 91,667 | 40,000 | 8,100 | (5) | — | 139,767 | ||||||||||||||
| Chief Financial Officer |
2008 | 40,000 | 18,000 | 12,900 | — | 70,900 | |||||||||||||||
| Peter G. Piferi |
2009 | 250,000 | 100,000 | — | 2,860 | 352,860 | |||||||||||||||
| Chief Operating Officer |
2008 | 250,000 | 75,000 | — | 2,609 | 327,609 | |||||||||||||||
| Oscar L. Thomas |
2009 | 175,000 | 130,000 | (6) | — | 5,355 | 310,355 | ||||||||||||||
| Vice President, Business Affairs |
2008 | 122,051 | 59,750 | 120,000 | 3,005 | 304,812 | |||||||||||||||
| Michael M. Moore |
2009 | 173,750 | 48,500 | (7) | — | 260 | 222,510 | ||||||||||||||
| Vice President, Operations |
2008 | 37,548 | 23,500 | 21,300 | 12 | 82,354 | |||||||||||||||
| (1) | Amounts represent grant date fair value of the option awards computed in accordance with ASC Topic 718. For a discussion of the assumptions made in the valuation of the awards, see note 2 to the financial statements included elsewhere in this prospectus and the discussion under “Management’s Discussion and Analysis of Financial Condition and Results of Operations—Critical Accounting Policies and Significant Judgements and Estimates—Share-based compensation”. |
| (2) | These amounts consist of the group medical, life and disability premiums paid by us. |
| (3) | Represents the grant date fair value in accordance with ASC Topic 718 for: (a) an option to purchase 2,500 shares of our common stock issued to Mr. Jenkins on December 10, 2009 ($8,100); and (b) an option to purchase 66,652 shares of our common stock issued to Mr. Jenkins on December 22, 2009 ($183,960). |
| (4) | In September 2004, Mr. Jenkins purchased 500,000 shares of our common stock, which he paid for by delivering to us a non-recourse promissory note. Section 402(a) of the Sarbanes-Oxley Act required that the note be repaid prior to the filing of our registration statement for the initial public offering of our common stock. Our Board of Directors formed a special committee of independent directors to review and evaluate any potential transaction with Mr. Jenkins with respect to his loan. The special committee approved, and our Board of Directors ratified, a transaction pursuant to which, on December 22, 2009, Mr. Jenkins sold us 66,652 shares of common stock valued at $9.64 per share and we issued to Mr. Jenkins an option to purchase 66,652 shares of common stock with an exercise price of $9.64 per share. Our Board of Directors determined that the fair market value of our common stock as of December 22, 2009 was $9.64 per share. We paid most of the stock purchase price for Mr. Jenkins’ shares by cancelling Mr. Jenkins’ promissory note and we paid the remaining portion of approximately $47,833 in cash. See “Certain Relationships and Related Party Transactions – Related Person Transactions.” The purpose of the transaction was to satisfy Mr. Jenkins’ promissory note to enable us to file our registration statement for the initial public offering of our common stock while maintaining as closely as possible the original economics of Mr. Jenkins’ loan transaction. The December 22, 2009 stock option we issued to Mr. Jenkins, when computed in accordance with ASC Topic 718, resulted in $183,960 of non-cash compensation to Mr. Jenkins. |
| (5) | Represents the grant date fair value of the option award computed in accordance with ASC Topic 718 for an option to purchase 2,500 shares of our common stock issued to Mr. Thomas on December 10, 2009. |
| (6) | This bonus amount includes non-discretionary quarterly bonuses totaling $50,000, which was paid pursuant to Mr. Thomas’ employment letter. |
| (7) | This bonus amount includes a non-discretionary one-time bonus of $13,500, which was paid pursuant to Mr. Moore’s employment letter. |
105
Grants of Plan-Based Awards
The table below sets forth information concerning grants of plan based awards in 2009 to our named executive officers.
| Name |
Grant Date | All Other Option Awards: Number of Securities Underlying Options |
Exercise Price Of Option Awards(1) |
Grant Date Fair Value of Option Awards | |||||||
| Kimble L. Jenkins |
December 10, 2009 | 2,500 | (2) | $ | 9.64 | $ | 8,100 | ||||
| December 22, 2009 | 66,652 | (3) | 9.64 | 183,960 | |||||||
| John C. Thomas, Jr. |
December 10, 2009 | 2,500 | (2) | 9.64 | 8,100 | ||||||
| (1) | The exercise price of each stock option granted to our named executive officers is equal to the fair market value of one share of the underlying common stock on the grant date. |
| (2) | This option was awarded following our 2009 annual meeting of our stockholders in connection with the recipient’s service as a director. These options vest on the earlier to occur of: (a) the one year anniversary of the grant date; or (b) the day immediately preceding the 2010 annual meeting of stockholders. |
| (3) | The shares subject to this option will vest ratably on the first, second and third anniversaries of the grant date, December 22, 2010, December 22, 2011 and December 22, 2012. |
All the stock options granted to the named executive officers were granted under our 2007 Stock Incentive Plan. The compensation committee, which administers our 2007 Stock Incentive Plan, has general authority to accelerate, extend, or otherwise modify the benefits under the stock options in certain circumstances within overall plan and other limitations. The compensation committee has no present intention to exercise that authority with respect to these stock options.
Outstanding Equity Awards at December 31, 2009
The table below sets forth information regarding the outstanding equity awards held by our named executive officers at December 31, 2009.
| Option Awards | |||||||||||
| Name |
Number of Securities Underlying Unexercised Options (#) Exercisable |
Number of Securities Underlying Unexercised Options (#) Unexercisable |
Option Exercise Price ($) |
Option Expiration Date | |||||||
| Kimble L. Jenkins |
96,625 | (1) | — | (1) | $ | 3.20 | December 1, 2011 | ||||
| 5,000 | (2) | — | (2) | 3.20 | March 28, 2017 | ||||||
| 2,500 | (3) | — | (3) | 9.64 | September 16, 2018 | ||||||
| 2,500 | (4) | — | (4) | 9.64 | November 8, 2018 | ||||||
| — | (5) | 2,500 | (5) | 9.64 | December 10, 2019 | ||||||
| — | (6) | 66,652 | (6) | 9.64 | September 1, 2013 | ||||||
| John C. Thomas, Jr. |
100,000 | (7) | — | (7) | 0.88 | April 12, 2014 | |||||
| 5,000 | (2) | — | (2) | 3.20 | March 28, 2017 | ||||||
| 2,500 | (3) | — | (3) | 9.64 | September 16, 2018 | ||||||
| 2,500 | (4) | — | (4) | 9.64 | November 8, 2018 | ||||||
| — | (5) | 2,500 | (5) | 9.64 | December 10, 2019 | ||||||
| Peter G. Piferi |
75,000 | (8) | — | (8) | 3.20 | December 1, 2017 | |||||
| Oscar L. Thomas |
20,833 | (9) | 41,667 | (9) | 6.04 | April 30, 2018 | |||||
| Michael M. Moore |
2,500 | (10) | 5,000 | (10) | 9.64 | November 7, 2018 | |||||
| (1) | This warrant was immediately exercisable on the date of grant, December 1, 2006. |
| (2) | The vesting of shares subject to this option occurred on the date of grant, March 28, 2007. |
106
| (3) | The vesting of shares subject to this option occurred on the date of grant, September 16, 2008. |
| (4) | The vesting of shares subject to this option occurred on the first anniversary of the date of grant, November 8, 2009. |
| (5) | The vesting of shares subject to this option occured on April 22, 2010, which was the day immediately preceding the 2010 annual meeting of our stockholders. |
| (6) | The shares subject to this option vest ratably on the first, second and third anniversaries of the grant date, December 22, 2010, December 22, 2011 and December 22, 2012. |
| (7) | The vesting of shares subject to this option occurred on the date of grant, April 12, 2004. |
| (8) | One-third of the shares subject to this option vested upon the first anniversary of Mr. Piferi’s hire date, December 1, 2007, one-third vested on the second anniversary, December 1, 2008, and the remaining one-third vested on the third anniversary, December 1, 2009. |
| (9) | One-third of the shares subject to this option vested on the first anniversary of Mr. Thomas’ hire date, April 18, 2009. The remaining shares subject to this option vest ratably on the second and third anniversaries of Mr. Thomas’ hire date, April 18, 2010 and April 18, 2011. |
| (10) | One-third of the shares subject to this option vested on the first anniversary of Mr. Moore’s hire date, October 9, 2009. The remaining shares subject to this option vest ratably on the second and third anniversaries of Mr. Moore’s hire date, October 9, 2010 and October 9, 2011. |
Option Exercises
None of our named executive officers exercised stock options in 2009.
Employment Agreements and Potential Payments Upon Termination or Change of Control
In June 2010, we entered into employment agreements with each of our named executive officers other than Mr. John C. Thomas, Jr. Mr. Thomas ceased serving as our Chief Financial Officer in April 2010, and we entered into a separation agreement with Mr. Thomas in April 2010, which is summarized below under “— John C. Thomas, Jr. Separation Agreement”. In June 2010, we also entered into an employment agreement with Mr. David W. Carlson, our new Chief Financial Officer. We have included a summary of potential payments due to Mr. Carlson upon termination or change of control even though Mr. Carlson is not a named executive officer. The employment agreements will become effective upon completion of this offering. The material terms of each executive’s employment agreement are set forth in the following table and the discussion below:
| Executive |
Initial Term(1) | Salary(2) | Bonus | ||||
| Kimble L. Jenkins |
5 years | $ | 325,000 | (3) | |||
| Peter G. Piferi |
3 years | $ | 250,000 | (3) | |||
| David W. Carlson |
2 years | $ | 225,000 | (3) | |||
| Oscar L. Thomas |
2 years | $ | 225,000 | (3) | |||
| Michael M. Moore |
1 years | $ | 175,000 | (3) | |||
| (1) | The term of each executive’s employment agreement is subject to one year renewals at the end of the initial term. |
| (2) | Each executive’s salary is subject to adjustment at the discretion of our compensation committee, subject to certain limitations. |
| (3) | Each executive is eligible for a cash bonus in an amount and upon terms and conditions determined by our compensation committee. |
In addition, under each employment agreement, each executive is: (i) eligible for equity compensation in an amount and based upon goals and criteria determined by our compensation committee; (ii) entitled to participate in any benefit plan from time to time in effect for our executives and/or employees generally, subject to the eligibility provisions of that plan; and (iii) entitled to reimbursement for all reasonable and necessary business expenses incurred or paid in the performance of the executive’s duties. Additionally, each executive is subject to a confidentiality agreement and a non-compete agreement.
If the executive’s employment is terminated due to his death or permanent disability, then he will be entitled to receive: (i) any base salary and bonus compensation earned but unpaid as of the termination date; (ii) reimbursement of business expenses he incurred as of the termination date; and (iii) if properly elected and to the extent eligible, health care continuation coverage for himself, if applicable under the circumstances, and his spouse and dependents for up to 12 months. In addition, the executive will be entitled to receive any vested benefits under our award plans and benefit plans in accordance with the terms of those plans.
107
If we terminate the employment of any of Messrs. Jenkins, Piferi, Carlson or Thomas without cause or if any of Messrs. Jenkins, Piferi, Carlson or Thomas terminates his employment for good reason, as those terms are defined in his respective employment agreement, then such executive will receive: (i) any base salary and bonus compensation earned but unpaid as of the termination date; (ii) one and one-half times his base salary in effect on the termination date; (iii) one and one-half times his average bonus for the previous two years, if any; (iv) if properly elected and to the extent eligible, health care continuation coverage for himself, his spouse and his dependents for up to 18 months; and (v) reimbursement of business expenses he incurred as of the termination date. If we terminate Mr. Moore’s employment without cause or if Mr. Moore terminates his employment for good reason, as those terms are defined in his employment agreement, then Mr. Moore will receive: (i) any base salary and bonus compensation earned but unpaid as of the termination date; (ii) one times his base salary in effect on the termination date; (iii) one times his average bonus for the previous two years, if any; (iv) if properly elected and to the extent eligible, health care continuation coverage for himself, his spouse and his dependents for up to 12 months; and (v) reimbursement of business expenses he incurred as of the termination date. In addition, under each employment agreement, if we terminate the employment of the executive without cause or executive terminates his employment for good reason, any unvested stock options and restricted stock previously granted to the executive will become fully vested on the termination date.
Upon a change of control, any unvested stock options and restricted stock previously granted to the executives will become fully vested. In addition, if we terminate the employment of any of Messrs. Jenkins, Piferi, Carlson or Thomas without cause, or if any of Messrs. Jenkins, Piferi, Carlson or Thomas terminates his employment for good reason, in either case within four months prior to or within 12 months following a change of control, then he will be entitled to receive a lump sum payment equal to: (i) any base salary and bonus compensation earned but unpaid as of the termination date; (ii) two times his base salary in effect on the termination date; (iii) two times the greater of his average bonus for the previous two years or his current year target bonus, if any; (iv) an amount equal to the premium for 24 months of health care continuation coverage for himself, his spouse and his dependents, to the extent eligible for such coverage; and (v) reimbursement of business expenses he incurred as of the termination date. If we terminate Mr. Moore’s employment without cause, or if Mr. Moore terminates his employment for good reason, in either case within four months prior to or within 12 months following a change of control, then Mr. Moore will be entitled to receive a lump sum payment equal to: (i) any base salary and bonus compensation earned but unpaid as of the termination date; (ii) one times his base salary in effect on the termination date; (iii) the greater of his average bonus for the previous two years or his current year target bonus, to the extent eligible for such coverage; (iv) an amount equal to the premium for 12 months of health care continuation coverage for himself, his spouse and his dependents, if any; and (v) reimbursement of business expenses he incurred as of the termination date.
The following table includes estimates of the potential payments that we would be required to make and the value resulting from the acceleration of stock options in each of the circumstances described above. Our estimates are based are the following general assumptions:
| • | The executive entered into his employment agreement as described above prior to December 31, 2009; |
| • | The date of termination is December 31, 2009; |
| • | The executive’s base salary as of the date of termination is the initial base salary set forth in his employment agreement; |
| • | There is no accrued and unpaid salary or bonus compensation as of the date of termination; and |
| • | There is no unpaid reimbursement for business expenses incurred as of the date of termination. |
108
| Executive |
Benefit |
Death and Disability Termination |
Termination Without Cause and Termination for Good Reason |
Change of Control(1) |
Change
of Control Termination(2) | ||||||||
| Kimble L. Jenkins |
Salary |
— | $ | 487,500 | — | $ | 650,000 | ||||||
| Bonus |
— | $ | 56,250 | — | $ | 75,000 | |||||||
| Benefits continuation |
$ | 6,480 | $ | 9,720 | — | $ | 12,960 | ||||||
| Stock option acceleration(3) |
— | 24,895 | 24,895 | 24,895 | |||||||||
| Total |
$ | 6,480 | 578,365 | 24,895 | 762,855 | ||||||||
| Peter G. Piferi |
Salary |
— | $ | 375,000 | — | $ | 500,000 | ||||||
| Bonus |
— | $ | 93,750 | — | $ | 125,000 | |||||||
| Benefits continuation |
$ | 2,556 | $ | 3,834 | — | $ | 5,112 | ||||||
| Stock option acceleration(3) |
— | — | — | — | |||||||||
| Total |
$ | 2,556 | $ | 472,854 | — | $ | 630,112 | ||||||
| David W. Carlson |
Salary |
— | $ | 337,500 | — | $ | 450,000 | ||||||
| Bonus |
— | — | — | — | |||||||||
| Benefits continuation |
$ | 6,480 | $ | 9,720 | — | $ | 12,960 | ||||||
| Stock option acceleration(3) |
— | — | — | — | |||||||||
| Total |
$ | 6,480 | $ | 347,220 | — | $ | 462,960 | ||||||
| Oscar L. Thomas |
Salary |
— | $ | 337,500 | — | $ | 450,000 | ||||||
| Bonus |
— | $ | 44,813 | — | $ | 59,750 | |||||||
| Benefits continuation |
$ | 6,480 | $ | 9,720 | — | $ | 12,960 | ||||||
| Stock option acceleration(3) |
— | 165,000 | 165,000 | 165,000 | |||||||||
| Total |
$ | 6,480 | 557,033 | 165,000 | 687,710 | ||||||||
| Michael M. Moore |
Salary |
— | $ | 175,000 | — | $ | 175,000 | ||||||
| Bonus |
— | $ | 11,750 | — | $ | 11,750 | |||||||
| Benefits continuation |
— | — | — | ||||||||||
| Stock option acceleration(3) |
— | 1,800 | 1,800 | 1,800 | |||||||||
| Total |
— | 188,550 | 1,800 | 188,550 | |||||||||
| (1) | In the event of a change of control, the executive’s unvested restricted stock and stock options will become immediately vested and exercisable regardless of whether the executive is terminated in connection with the change of control. |
| (2) | With respect to a change of control termination, the value of the stock option acceleration is included for clarity of presentation; however, the options vest upon the change of control regardless of whether the executive is terminated. See the footnote above. |
| (3) | Assumes triggering event effective as of December 31, 2009 and excludes vested options and stock held as of such date. There was no public market for our common stock in 2009. We have estimated the market value of the accelerated stock options based on the difference between our assumed initial public offering price of $10.00 per share, which is the mid-point of the range listed on the cover of this prospectus, and the exercise price of such accelerated options. |
For purposes of these benefits, a change of control is deemed to occur, in general, if there is: (1) a change in our ownership; (2) a change in our effective control; or (3) a change in the ownership of a substantial portion of our assets. For purposes of this definition, a change in our ownership will occur on the date on which any one person, or more than one person acting as a group, acquires ownership of our stock that, together with stock already held by such person or group, constitutes more than 50% of the total fair market value or total voting power of our stock. A change in our effective control will occur on the date on which either (i) a person, or more than one person acting as a group, acquires ownership of our stock possessing 30% or more of the total voting power of our stock, taking into account all such stock acquired during the 12-month period ending on the date of the most recent acquisition, or (ii) a majority of the members of our Board of Directors is replaced during any 12-month period by directors whose appointment or election is not endorsed by a majority of the members of our
109
Board of Directors prior to the date of the appointment or election. A change in the ownership of a substantial portion of our assets will occur on the date on which any one person, or more than one person acting as a group, other than a person or group of persons that is related to us, acquires assets from us that have a total gross fair market value equal to or more than 40% of the total gross fair market value of all of our assets immediately prior to such acquisition or acquisitions, taking into account all such assets acquired during the 12-month period ending on the date of the most recent acquisition.
John C. Thomas, Jr. Separation Agreement
In April 2010, we entered into a separation agreement with Mr. John C. Thomas, Jr., who previously served as our Chief Financial Officer. Under the separation agreement, Mr. Thomas ceased to be our employee, we agreed to pay Mr. Thomas severance totaling $87,000, and Mr. Thomas agreed to consult and cooperate with us in connection with the orderly transition of his business responsibilities to our new Chief Financial Officer.
Benefit Plans
1998 Stock Option Plan
We adopted the 1998 Stock Option Plan on June 24, 1998 to enable us to attract, retain and motivate our officers, directors, employees and consultants. Of the 375,000 shares of common stock that were eligible for issuance pursuant to awards made under this plan, 292,500 shares of common stock were subject to options outstanding as of March 31, 2010. As of such date, the outstanding options had a weighted average exercise price of $1.28 per share and had expiration dates ranging from January 1, 2011 to October 21, 2014. We terminated this plan, effective June 24, 2008, with respect to future grants such that no new options may be awarded under this plan.
2007 Stock Incentive Plan
We adopted the 2007 Stock Incentive Plan on March 28, 2007 to enable us to attract, retain and motivate our officers, directors, employees and consultants. Of the 625,000 shares of common stock that were eligible for issuance pursuant to awards made under this plan, 308,125 shares of common stock were subject to options outstanding as of March 31, 2010. As of such date, the outstanding options had a weighted average exercise price of $5.82 per share and had expiration dates ranging from March 28, 2017 to December 10, 2019. Although this plan remains in effect and options under the plan remain outstanding, we ceased making awards under the plan upon the adoption of our 2010 Incentive Compensation Plan.
2010 Incentive Compensation Plan
We adopted the 2010 Incentive Compensation Plan, or the 2010 Plan, on April 23, 2010. The principal purpose of the 2010 Plan is to attract, retain and motivate selected employees, consultants and directors through the granting of stock-based compensation awards and cash-based performance bonus awards. The 2010 Plan is also designed to permit us to make cash-based awards and equity-based awards intended to qualify as “performance-based compensation” under Section 162(m) of the Code.
This following summary is qualified in its entirety by reference to the text of the 2010 Plan, which is filed as an exhibit to the registration statement of which this prospectus is a part.
Eligibility. Awards may be granted under the 2010 Plan to officers, directors (including non-employee directors) and other employees of our company or any of our subsidiaries or other affiliates, to any individual who is an advisor, consultant or other provider of services to us or any of our subsidiaries or other affiliates and to any other individuals who are approved by our Board of Directors as eligible to participate in the plan. Only our employees or those of any of our subsidiaries are eligible to receive incentive stock options.
110
Administration, Amendment and Termination. Our compensation committee will have the power and authority to administer the 2010 Plan. The compensation committee will have the authority to interpret the terms and intent of the 2010 Plan, determine eligibility for and terms of awards for participants and make all other determinations necessary or advisable for the administration of the 2010 Plan. To the extent permitted by law, our compensation committee may delegate authority under the 2010 Plan to our Chief Executive Officer or to our other executive officers under conditions and limitations the compensation committee may establish.
The compensation committee may amend, suspend or terminate the 2010 Plan at any time with respect to any shares of common stock as to which awards have not been made. No such action may amend the 2010 Plan without the approval of stockholders if the amendment is required to be submitted for stockholder approval by applicable law, rule or regulation.
Awards. Awards under the 2010 Plan may be made in the form of: options, SARs, stock awards, restricted share units, cash bonuses or other incentive award granted under the 2010 Plan, whether singly, in combination, or in tandem. Any of the foregoing awards may be made subject to attainment of performance goals over any applicable performance period.
Shares Subject to the Plan. The aggregate number of shares of our common stock that may be issued initially pursuant to stock awards under the 2010 Plan is 1,250,000 shares. In connection with this offering, we intend to (i) grant options under the 2010 Plan to purchase 541,000 shares of common stock with exercise prices equal to the initial public offering price and (ii) issue 30,000 shares of common stock under the 2010 Plan, assuming an initial public offering price of $10.00 per share, which is the mid-point of the range listed on the cover of this prospectus. The maximum number of shares that may be issued pursuant to the exercise of incentive stock options under the 2010 Plan is 625,000. Shares issued under the 2010 Plan may be authorized but unissued shares or treasury shares. Any shares covered by an award, or portion of an award, granted under the 2010 Plan that is forfeited or canceled, expires or is settled in cash will be deemed not to have been issued for purposes of determining the maximum number of shares available for issuance under the 2010 Plan.
Adjustment of Shares Subject to 2010 Plan. In the event of certain changes in our capitalization, the compensation committee will adjust, among other award terms, the number and kind of shares or property that may be delivered in connection with awards and the exercise price, grant price or purchase price relating to any award in such manner as the compensation committee determines to be necessary to prevent dilution or enlargement of the rights of participants.
Effect of a Change of Control. Upon the occurrence of a change of control, the compensation committee may:
| • | accelerate, vest or cause the restrictions to lapse with respect to all or any portion of an award under the 2010 Plan; |
| • | cancel such awards for fair value (as determined by the compensation committee); |
| • | provide for the issuance of substitute awards that will substantially preserve the otherwise applicable terms of any affected awards previously granted under the 2010 Plan, as determined by the compensation committee; or |
| • | provide that for a period of at least 10 days prior to the change of control, option awards will be exercisable as to all shares of common stock subject thereto and that upon the occurrence of the change of control, such awards will terminate and be of no further force or effect. |
Corporate Performance Objectives. Section 162(m) of the Code limits public companies to an annual deduction for federal income tax purposes of $1,000,000 for compensation paid to their Chief Executive Officer and, based on recent IRS interpretation, the three most highly compensated executive officers determined at the end of each year. Performance-based compensation is excluded from this limitation. The 2010 Plan is designed to permit the compensation committee to grant awards that qualify as performance-based for purposes of satisfying the conditions of Section 162(m) at such time as the 2010 Plan becomes subject to Section 162(m).
111
Key Personnel Incentive Program
We have adopted the Key Personnel Incentive Program, or the program, to provide a key employee and consultant with the opportunity to receive incentive bonus payments based on future performance of services to the company or upon a consummation of a sale transaction, as defined in the program. The compensation committee of our Board of Directors is responsible for administering the program, and the only participants in the program are Paul A. Bottomley and Parag Karmarkar. The program will terminate on the earlier of December 31, 2015 or the occurrence of a sale transaction.
Service Bonuses
Until the occurrence of a sale transaction, each participant will be entitled to receive semi-annual service bonuses beginning on June 30, 2012 and continuing through December 31, 2015 if the participant continues to provide services to us as our consultant or employee as of the respective payment dates. Pursuant to their awards, Dr. Bottomley and Mr. Karmarkar would receive service bonuses totaling up to $1,700,000 and $1,000,000, respectively, payable in eight equal semi-annual installments. If the participant’s consultancy or employment is (i) terminated due to the participant’s death or disability, or (ii) involuntarily terminated by us other than for cause, as defined in the program, then the participant will be deemed vested, as of the termination date, in all future service bonus payments, and we will pay that aggregate amount no later than March 15 of the year following the year in which the termination occurred.
Bonus Upon a Sale Transaction
In the event of a sale transaction, each of the participants will be entitled to receive a bonus payment under the program if the participant continues to provide services to us as our consultant or employee as of the date of the transaction. Mr. Karmarkar would receive a bonus equal to $1,000,000, less any service bonus payments made to Mr. Karmarkar as described above. Dr. Bottomley would receive a bonus equal to (i) $1,000,000, plus (ii) 1.4% of the amount by which the “net proceeds” from the sale transaction exceed $50,000,000, but not to exceed $700,000, less (iii) any service bonus payments made to Dr. Bottomley as described above. Following a sale transaction, neither participant will be entitled to receive any further service bonuses.
For purposes of the program, the “net proceeds” from a sale transaction will be the portion of the aggregate cash and non-cash consideration paid or payable in connection with the consummation of the sale transaction that is distributed, or otherwise available for distribution, to holders of our common stock.
Cardiac EP Business Participation Plan
We have adopted the Cardiac EP Business Participation Plan, or the plan, to enable us to provide a key product development advisor and consultant with financial rewards in the event that we sell our business operations relating to catheter-based MRI-guided cardiac ablation to treat cardiac arrhythmias, which we refer to as our cardiac EP business operations. The cardiac EP business operations include our operations relating to the ClearTrace system for MRI-guided cardiac ablation to treat cardiac arrhythmias, but it does not include our operations relating to our ClearPoint system, our SafeLead Development Program or any other product or product candidate. The sole participant in the plan is Dr. Nassir F. Marrouche.
In the event that we sell our cardiac EP business operations, whether on a stand-alone basis or as part of the sale of our entire company, the participant will receive a payment under the plan equal to (i) the transaction value paid for or allocated to the cardiac EP business operations in the sale, multiplied by (ii) the participant’s “participation interest” at the time of the sale. The participant was initially awarded a participation interest of 6.6%. However, that percentage interest will be equitably reduced from time to time to take into account any equity financing transactions, including this offering, in which we issue shares of our common stock or securities convertible into shares of our common stock in exchange for cash proceeds. The plan will terminate on June 2, 2025.
112
401(k) Plan
We offer a 401(k) Plan pursuant to Section 401(k) of the Code. All full time United States employees are eligible to participate in the plan. The plan permits pretax contributions by participants not to exceed annual amounts allowable under the Code. Participants are fully vested in their contributions.
Limitations on Directors’ Liability and Indemnification Agreements
As permitted by Delaware law, we have adopted provisions in our certificate of incorporation and bylaws, both of which will become effective upon the completion of this offering, that limit or eliminate the personal liability of directors for a breach of their fiduciary duty of care as a director. The duty of care generally requires that, when acting on behalf of the corporation, a director exercise an informed business judgment based on all material information reasonably available to him or her. Consequently, a director will not be personally liable to us or our stockholders for monetary damages or breach of fiduciary duty as a director, except for liability for any:
| • | breach of the director’s duty of loyalty to us or our stockholders; |
| • | act or omission not in good faith or that involves intentional misconduct or a knowing violation of law; |
| • | act related to unlawful stock repurchases, redemptions or other distributions or payments of dividends; or |
| • | transaction from which the director derived an improper personal benefit. |
These limitations of liability do not limit or eliminate our rights or any stockholder’s rights to seek non-monetary relief, such as injunctive relief or rescission. These provisions will not alter a director’s liability under federal securities laws. Our certificate of incorporation that will become effective upon the completion of this offering also authorizes us to indemnify our officers, directors and other agents to the fullest extent permitted under Delaware law.
As permitted by Delaware law, our bylaws also provide that:
| • | we will indemnify our directors, officers, employees and other agents to the fullest extent permitted by law; |
| • | we may advance expenses to our directors, officers, employees and other agents in connection with a legal proceeding to the fullest extent permitted by law; and |
| • | the rights provided in our bylaws are not exclusive. |
We believe that indemnification under our bylaws covers at least negligence and gross negligence on the part of indemnified parties. Our bylaws also permit us to secure insurance on behalf of any officer, director, employee or other agent for any liability arising out of his or her actions in connection with their services to us, regardless of whether our bylaws permit such indemnification. We have obtained such insurance.
In addition to the indemnification provided for in our certificate of incorporation and bylaws, we intend to enter into separate indemnification agreements with each of our directors and executive officers. These indemnification agreements may require us, among other things, to indemnify our directors and officers for some expenses, including attorneys’ fees, judgments, fines and settlement amounts incurred by a director or officer in any action or proceeding arising out of his or her service as one of our directors or officers, or any of our subsidiaries or any other company or enterprise to which the person provides services at our request. We believe that these provisions and agreements are necessary to attract and retain qualified individuals to serve as directors and officers. There is no pending litigation or proceeding involving any of our directors or officers to which indemnification is required or permitted, and we are not aware of any threatened litigation or proceeding that may result in a claim for indemnification.
113
CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS
Policies and Procedures for Related Person Transactions
We have adopted a related person transactions policy, to be effective upon completion of this offering, pursuant to which our executive officers, directors and principal stockholders, including their immediate family members, are not permitted to enter into a related person transaction with us without the consent of our audit committee. Any request for us to enter into a transaction with an executive officer, director, principal stockholder or any of such persons’ immediate family members, in which the amount involved exceeds $120,000 must be presented to our audit committee for review, consideration and approval. All of our directors, executive officers and employees are required to report to our audit committee any such related person transaction. In approving or rejecting the proposed agreement, our audit committee will take into account, among other factors it deems appropriate, whether the proposed related person transaction is on terms no less favorable than terms generally available to an unaffiliated third party under the same or similar circumstances, the extent of the person’s interest in the transaction and, if applicable, the impact on a director’s independence. After consideration of these and other factors, the audit committee may approve or reject the transaction. Under the policy, if we should discover related person transactions that have not been approved, the audit committee will be notified and will determine the appropriate action, including ratification, rescission or amendment of the transaction.
Related Person Transactions
The following is a description of transactions since January 1, 2009 to which we have been a party, in which the amount involved in the transaction exceeds $120,000, and in which any of our executive officers, directors and principal stockholders, including their immediate family members, had or will have a direct or indirect material interest.
In September 2004, Mr. Jenkins, our Chief Executive Officer, purchased 500,000 shares of our common stock for an aggregate purchase price of $480,000. Mr. Jenkins paid the purchase price by delivering to us a non-recourse promissory note in the principal amount of $480,000, and Mr. Jenkins pledged the purchased shares as security for the note. The note was amended and restated on September 30, 2008 to extend the maturity date. As of December 22, 2009, the outstanding balance on the note was $594,687 (including $114,687 of accrued interest). Section 402(a) of the Sarbanes-Oxley Act required that the note be repaid prior to the filing of our registration statement for the initial public offering of our common stock. Our Board of Directors formed a special committee of independent directors to review and evaluate any potential transaction with Mr. Jenkins with respect to his loan. The special committee approved, and our Board of Directors ratified, a transaction pursuant to which, on December 22, 2009, Mr. Jenkins sold us 66,652 shares of common stock valued at $9.64 per share and we issued to Mr. Jenkins an option to purchase 66,652 shares of common stock with an exercise price of $9.64 per share. Our Board of Directors determined that the fair market value of our common stock as of December 22, 2009 was $9.64 per share. We paid a portion of the stock purchase price, approximately $594,687, by cancelling Mr. Jenkins’ promissory note and the remainder, approximately $47,833, was paid in cash. The purpose of the transaction was to satisfy Mr. Jenkins’ promissory note to enable us to file of our registration statement for the initial public offering of our common stock while maintaining as closely as possible the original economics of Mr. Jenkins’ loan transaction.
Between January 2006 and August 2007, Boston Scientific, one of our 5% common stockholders and the employer of one of our directors, loaned us $1,500,000 in six equal quarterly installments pursuant to a convertible promissory note. This note matured on June 30, 2008, at which time Boston Scientific converted the note into 417,960 shares of our common stock and a warrant to purchase 417,960 shares of our common stock, which warrant has since expired. As such, we have no remaining obligations under the note.
On January 30, 2009, we repurchased 125,000 shares of our common stock from DARA, one of our 5% common stockholders, for $500,000. In connection with this repurchase, we also loaned $500,000 to DARA pursuant to a secured promissory note having an interest rate of 8% per year and a maturity date of July 31, 2010.
114
The secured promissory note was collateralized by 125,000 shares of our common stock held by DARA. On December 31, 2009, DARA repaid the $500,000 loan plus accrued interest of $36,712 by tendering to us an additional 134,178 shares of our common stock in full satisfaction of the note.
During 2009, Boston Scientific loaned us $3,500,000 pursuant to the terms of three convertible promissory notes. Interest on the loans accrues at 10% per year and compounds annually. The Boston Scientific loans are secured by a first priority security interest in all of our assets. Each loan matures on the second anniversary of the date on which the funds were advanced. In addition, we will be required to prepay all or a portion of loans upon the consummation of any qualified financing, which is any equity financing in which shares of our preferred stock are issued in exchange for cash proceeds. Upon consummation of a qualified financing from Medtronic, Inc., St. Jude Medical, Inc., or Johnson & Johnson, or any of their respective subsidiaries or affiliates, up to 100% of the cash proceeds from such qualified financing must be used to prepay the outstanding principal of the loans and accrued interest thereon. Upon consummation of a qualified financing from any other investor, up to 25% of the cash proceeds from such qualified financing must be applied by us to prepay the outstanding principal of the loans and accrued interest thereon. We can repay each loan at anytime prior to its respective maturity date. At the option of Boston Scientific, these loans are convertible into one share of our common stock for every $8.00 of principal and interest outstanding at the time of conversion. To the extent that Boston Scientific has not exercised its conversion right prior to the completion of this offering, Boston Scientific will no longer have the right to convert the notes into shares of stock.
In addition to the disclosure above, the terms of the Key Personnel Incentive Plan, which is more fully described in the section entitled “Benefit Plans—Key Personnel Incentive Plan”, is incorporated and restated herein.
Third Amended and Restated Investors Rights’ Agreement
Pursuant to our Third Amended and Restated Investors Rights’ Agreement, or Rights Agreement, certain of our stockholders and their affiliates and transferees have registration rights. Pursuant to the Rights Agreement, holders of registrable shares may require us, on not more than two occasions at any time beginning six months from the date of the closing of this offering, to file a registration statement under the Securities Act to register for resale their shares of common stock. As of April 30, 2010, the holders of approximately 5,800,000 shares of our common stock and common stock issuable upon conversion of our preferred stock had registration rights pursuant to the Rights Agreement. For more information concerning the Rights Agreement, please see “Description of Capital Stock—Registration Rights.”
Indemnification Agreements
Prior to this offering, we expect to enter into separate indemnification agreements with each of our directors and executive officers, in addition to the indemnification provided for in our certificate of incorporation and bylaws. See “Management—Limitations on Directors’ Liability and Indemnification Agreements”.
115
The following table sets forth information as of April 30, 2010 regarding the beneficial ownership of our common stock by:
| • | each person, or group of affiliated persons, who is known by us to own beneficially five percent or more of our common stock; |
| • | each of our directors; |
| • | each of our named executive officers; and |
| • | all our directors and executive officers as a group. |
Percentage ownership calculations for beneficial ownership before this offering are based on 7,629,405 shares outstanding as of April 30, 2010, assuming the conversion into common stock of all outstanding shares of our preferred stock and the bridge notes. Percentage ownership calculations for beneficial ownership after this offering are based on 10,129,405 shares outstanding after this offering, assuming no exercise of the underwriters’ over-allotment option, which includes the issuance of 2,500,000 shares in this offering. Shares beneficially owned before and after this offering reflect a 1-for-4 reverse stock split to be effected prior to completion of this offering, an assumed initial public offering price of $10.00 per share, which is the mid-point of the range listed on the cover of this prospectus, and accrued interest on convertible promissory notes through April 30, 2010.
Each individual or entity shown on the table has furnished information with respect to beneficial ownership. Except as otherwise indicated below, the address of each officer, director and five percent stockholder listed below is c/o SurgiVision, Inc., One Commerce Square, Suite 2550, Memphis, TN 38103.
We have determined beneficial ownership in accordance with the rules of the Securities and Exchange Commission. These rules generally attribute beneficial ownership of securities to persons who possess sole or shared voting power or investment power with respect to those securities. In addition, the rules include shares of common stock issuable pursuant to the exercise of stock options and warrants that are either immediately exercisable or exercisable within 60 days of April 30, 2010. These shares are deemed to be outstanding and beneficially owned by the person holding those options for the purpose of computing the percentage ownership of that person, but they are not treated as outstanding for the purpose of computing the percentage ownership of any other person. Unless otherwise indicated, the persons or entities identified in this table have sole voting and investment power with respect to all shares shown as beneficially owned by them.
116
| Beneficial Owner (Name and Address) |
Number of Shares Owned |
Percentage of Shares Outstanding | ||||||
| Before Offering | After Offering | |||||||
| 5% Stockholders |
||||||||
| Entities affiliated with Boston Scientific Neuromodulation Corporation(1) One Boston Scientific Plaza Natick, MA 01760 |
885,340 |
10.9 |
% |
8.4 |
% | |||
| Bruce Conway(2) 5514 Wenonah Dr Dallas, TX 75209 |
527,185 | 6.9 | 5.2 | |||||
| DARA Pharmaceuticals, Inc.(3) 8601 Six Forks Road, Suite 160 Raleigh, NC 27615 |
504,565 | 6.6 | 4.9 | |||||
| Directors and Named Executive Officers |
||||||||
| Kimble L. Jenkins(4) |
543,723 | 7.0 | 5.3 | |||||
| John C. Thomas, Jr.(5) |
192,731 | 2.5 | 1.9 | |||||
| Lenox D. Baker(6) |
17,500 | * | * | |||||
| Paul A. Bottomley(7) |
122,166 | 1.6 | 1.2 | |||||
| Charles E. Koob(8) |
31,250 | * | * | |||||
| Wendelin C. Maners(9) |
8,750 | * | * | |||||
| James K. Malernee, Jr. |
— | * | * | |||||
| Michael A. Pietrangelo |
— | * | * | |||||
| John N. Spencer, Jr. |
— | * | * | |||||
| Peter G. Piferi(10) |
75,000 | 1.0 | * | |||||
| Oscar L. Thomas(11) |
41,667 | * | * | |||||
| Michael M. Moore(12) |
2,500 | * | * | |||||
| All executive officers and directors as a group (15 persons)(13) |
1,037,787 | 12.8 | 9.8 | |||||
| * | Represents beneficial ownership of less than 1% of our outstanding common stock. |
| (1) | Includes 458,630 shares issuable upon the conversion of convertible promissory notes, the outstanding balance of which, including principal and accrued interest, was approximately $3,669,040 as of April 30, 2010. Also includes 8,750 shares that Ms. Maners has the right to acquire through the exercise of options, which she holds for the benefit of Boston Scientific Neuromodulation Corporation. Pursuant to the terms of the Stockholders’ Agreement, which terminates upon closing of this offering, Boston Scientific Neuromodulaton Corporation designated Ms. Maners as their nominee to serve on our Board of Directors. Boston Scientific is a reporting company under the Exchange Act, the shares of which are traded on the New York Stock Exchange and are widely held. |
| (2) | Includes 6,250 shares jointly held with his spouse, 25,000 shares held solely by his spouse, 6,250 shares issuable upon the optional conversion of a bridge note in the principal amount of $50,000 and 20,661 shares of common stock in the aggregate owned by the Alden M. Conway Trust, the Chase T. Conway Trust, the Merritt Elizabeth Conway Trust, the Edna N. Conway Irrevocable Trust FBO Alden M. Conway, the Edna N. Conway Irrevocable Trust FBO Chase T. Conway and the Edna N. Conway Irrevocable Trust FBO Merritt Elizabeth Conway. Mr. Conway is the trustee of each of the aforementioned trusts and has voting and investment power of each trust’s shares, which are held in trust for the benefit of his children. |
| (3) | Includes 101,250 shares that DARA Pharmaceuticals, Inc. has the right to acquire through the exercise of warrants. DARA Pharmaceuticals, Inc. is a reporting company under the Exchange Act, the shares of which are traded on the Nasdaq Capital Market and are widely held. |
| (4) | Includes 96,625 shares that Mr. Jenkins has the right to acquire through the exercise of warrants and 12,500 shares that Mr. Jenkins has the right to acquire through the exercise of options. |
| (5) | Includes 18,185 shares held by a family limited partnership of which Mr. Thomas is the general partner, 91 shares owned by Mr. Thomas’ daughter, and 112,500 shares that Mr. Thomas has the right to acquire through the exercise of stock options. Does not include 182 shares beneficially owned by Mr. Thomas’ spouse of which Mr. Thomas disclaims beneficial ownership. |
| (6) | Includes 17,500 shares that Dr. Baker has the right to acquire through the exercise of options. |
| (7) | Includes 75,000 shares that Dr. Bottomley has the right to acquire through the exercise of options. |
| (8) | Includes 20,000 shares jointly held with his spouse and 8,750 shares that Mr. Koob has the right to acquire through the exercise of options. |
| (9) | Includes 8,750 shares that Ms. Maners has the right to acquire through the exercise of options. Ms. Maners holds her options for the benefit of Boston Scientific Neuromodulation Corporation. Pursuant to the terms of the Stockholders’ Agreement, which terminates upon closing of this offering, Boston Scientific Neuromodulaton Corporation designated Ms. Maners as its nominee to serve on our Board of Directors. |
| (10) | Includes 75,000 shares that Mr. Piferi has the right to acquire through the exercise of options. |
| (11) | Includes 41,667 shares that Mr. Thomas has the right to acquire through the exercise of options. |
| (12) | Includes 2,500 shares that Mr. Moore has the right to acquire through the exercise of options. |
| (13) | Includes 453,292 shares exercisable through the exercise of options or warrants and 18,185 shares held by an entity controlled by a director. |
117
The following description of our capital stock gives effect to the amendment and restatement of our certificate of incorporation and bylaws, which will occur before the closing of this offering, a 1-for-4 reverse stock split, which will occur before the closing of this offering, and the conversion of our preferred stock and bridge notes into 2,500,125 shares of common stock (assuming an initial public offering price of $10.00 per share, which is the mid-point of the range listed on the cover of this prospectus), which will occur upon the closing of this offering, as if such conversion had occurred on April 30, 2010.
Upon completion of this offering, our authorized capital stock will consist of 70,000,000 shares of common stock, $0.01 par value per share, and 5,000,000 shares of preferred stock, $0.01 par value per share.
Common Stock
Outstanding Shares
As of April 30, 2010, we had 5,129,280 shares of common stock outstanding and 7,965,000 shares of preferred stock issued and outstanding that are convertible into 1,991,250 shares of common stock and $4,071,000 of bridge notes that are convertible into 508,875 shares of common stock. As of April 30, 2010, we had approximately 500 stockholders, assuming the conversion of all outstanding shares of our preferred stock and bridge notes into shares of our common stock. In addition, as of April 30, 2010, options and warrants to purchase 1,103,263 shares of common stock were issued and outstanding and we had outstanding convertible promissory notes that were convertible into 458,630 shares of common stock. Based on our outstanding capital stock as of April 30, 2010, upon the completion of this offering, there will be 10,129,405 shares of common stock outstanding assuming an initial public offering price of $10.00 per share, which is the mid-point of the range listed on the cover of this prospectus, conversion of all preferred stock into 1,991,250 shares of common stock, conversion of the bridge notes into 508,875 shares of common stock, no exercise of the underwriters’ over-allotment option, no exercise of outstanding stock options or warrants and no conversion of outstanding convertible promissory notes (other than the bridge notes).
Voting Rights
Each holder of our common stock is entitled to one vote for each share on all matters submitted to a vote of the stockholders, including the election of directors. Under our certificate of incorporation and bylaws, our stockholders will not have cumulative voting rights. Because of this, the holders of a majority of the shares of common stock entitled to vote in any election of directors can elect all of the directors standing for election, if they should so choose.
Dividends
Subject to preferences that may be applicable to any then outstanding preferred stock, holders of common stock are entitled to receive ratably those dividends, if any, as may be declared from time to time by the Board of Directors out of legally available funds.
Liquidation
In the event of our liquidation, dissolution or winding up, holders of common stock will be entitled to share ratably in the net assets legally available for distribution to stockholders after the payment of all of our debts and other liabilities and the satisfaction of any liquidation preference granted to the holders of any outstanding shares of preferred stock.
Rights and Preferences
Holders of common stock have no preemptive, conversion or subscription rights, and there are no redemption or sinking fund provisions applicable to the common stock. The rights, preferences and privileges of the holders of common stock are subject to, and may be adversely affected by, the rights of the holders of shares of any series of preferred stock that we may designate in the future.
118
Fully Paid and Nonassessable
All of our outstanding shares of common stock are, and the shares of common stock to be issued pursuant to this offering will be, fully paid and nonassessable.
Preferred Stock
Upon the closing of this offering, the Board of Directors will have the authority, without further action by the stockholders, to issue up to 5,000,000 shares of preferred stock in one or more series, to establish from time to time the number of shares to be included in each such series, to fix the rights, preferences and privileges of the shares of each wholly unissued series and any qualifications, limitations or restrictions thereon, and to increase or decrease the number of shares of any such series, but not below the number of shares of such series then outstanding. The Board of Directors may authorize the issuance of preferred stock with voting or conversion rights that could adversely affect the voting power or other rights of the holders of the common stock. The issuance of preferred stock, while providing flexibility in connection with possible acquisitions and other corporate purposes, could, among other things, have the effect of delaying, deferring or preventing a change in control of SurgiVision and may adversely affect the market price of the common stock and the voting and other rights of the holders of common stock. Upon completion of this offering, no shares of preferred stock will be issued or outstanding.
Registration Rights
In 1998 shortly following our formation, some of our initial investors entered into an investor rights agreement with us, which, among other things, provided demand and piggyback registration rights. As our operations to date have been funded primarily through the sale of our equity securities, we have amended the investor rights agreement with each offering of equity securities to extend the respective rights thereunder to the new investors. The investor rights agreement was most recently amended in 2006 in connection with a preferred stock offering, and it remains in place as the Rights Agreement.
Demand and Form S-3 Registration Rights
Pursuant to the Rights Agreement, at any time beginning six months after the consummation of this offering, the holders of approximately 5,800,000 shares of our common stock, or registrable shares, will have the right to require us to register the registrable shares under the Securities Act under specified circumstances. We will not be required to effect a demand registration for 120 days following the effectiveness of a registration statement relating to an underwritten public offering of our securities. Under specified circumstances, we also have the right to defer filing of a requested registration statement for a period of not more than 120 days, which right may not be exercised more than twice during any period of 12 consecutive months. These registration rights are subject to additional conditions and limitations, including the right of the underwriters to limit the number of shares included in any such registration under certain circumstances.
If we are eligible to file a registration statement on Form S-3, each holder of registrable shares of our common stock has the right to demand that we file additional registration statements, including a shelf registration statement, for such holders on Form S-3. We will not be required to effect more than four demand registrations in total, of which no more than two may be required to be effected by us at any time after the second anniversary of this offering and then only on Form S-3.
Piggyback Registration Rights
Pursuant to the Rights Agreement, at any time beginning six months after the consummation of this offering, whenever we propose to file a registration statement under the Securities Act, other than with respect to a registration related to employee benefit plans, debt securities, or corporate reorganizations, the holders of
119
registrable shares are entitled to notice of the registration and have the right to include their registrable shares in such a registration. In addition, two of our warrant holders, pursuant to the terms of their respective warrants, have similar piggyback registration rights. As of April 30, 2010, the holders of approximately 5,940,000 shares of our common stock, common stock issuable on the exercise of warrants and common stock issuable upon conversion of our preferred stock, would have been entitled to notice of the registration and would have been entitled to include their shares of common stock in the registration statement. The underwriters of any underwritten offering will have the right to limit the number of shares having registration rights to be included in the registration statement.
Expenses of Registration
We are required to pay all expenses relating to any demand or piggyback registration, other than underwriting discounts and commissions.
Delaware Anti-Takeover Law and Certain Provisions of our Certificate of Incorporation and Bylaws
Delaware Law
We are governed by Section 203 of the Delaware General Corporation Law. In general, Section 203 prohibits a public Delaware corporation from engaging in a “business combination” with an “interested stockholder” for a period of three years after the date of the transaction in which the person became an interested stockholder, unless the business combination is approved in a prescribed manner. A “business combination” includes mergers, asset sales or other transactions resulting in a financial benefit to the stockholder. An “interested stockholder” is a person who, together with affiliates and associates, owns, or within three years, did own, 15% or more of the corporation’s outstanding voting stock. These provisions may have the effect of delaying, deferring or preventing a change in our control.
Certificate of Incorporation and Bylaw Provisions
Our certificate of incorporation that will become effective upon the completion of this offering:
| • | provides for a staggered Board of Directors; |
| • | permits our Board of Directors to issue shares of preferred stock, with any rights, preferences and privileges as they may designate, including the right to approve an acquisition or other change in our control; |
| • | provides that the authorized number of directors may be changed only by resolution of the Board of Directors; |
| • | provides that all vacancies, including newly created directorships, may, except as otherwise required by law, be filled by the affirmative vote of a majority of directors then in office, even if less than a quorum; |
| • | requires that any action to be taken by our stockholders must be effected at a duly called annual or special meeting of stockholders and not be taken by written consent; |
| • | provides that stockholders seeking to present proposals before a meeting of stockholders or to nominate candidates for election as directors at a meeting of stockholders must provide notice in writing in a timely manner, and also specify requirements as to the form and content of a stockholder’s notice; |
| • | does not provide for cumulative voting rights (therefore allowing the holders of a majority of the shares of common stock entitled to vote in any election of directors to elect all of the directors standing for election, if they should so choose); |
| • | provides that special meetings of our stockholders may be called only by the chairman of the Board of Directors, our Chief Executive Officer or by the Board of Directors pursuant to a resolution adopted by a majority of the total number of authorized directors; and |
120
| • | provides that stockholders will be permitted to amend our amended and restated bylaws only upon receiving at least 66 2/3% of the votes entitled to be cast by holders of all outstanding shares then entitled to vote generally in the election of directors, voting together as a single class. |
These and other provisions contained in our certificate of incorporation and bylaws could delay or discourage some types of transactions involving an actual or potential change in our control or change in our management, including transactions in which stockholders might otherwise receive a premium for their shares over then current prices, and may limit the ability of stockholders to remove current management or approve transactions that stockholders may deem to be in their best interests and, therefore, could adversely affect the price of our common stock.
Nasdaq Capital Market Listing
We have been approved by the Nasdaq Capital Market for quotation of our common stock under the trading symbol “SRGV”.
Transfer Agent and Registrar
The Transfer Agent and Registrar for our common stock is Continental Stock Transfer and Trust Company. The transfer agent’s address is 17 Battery Place, New York, New York 10004 and its telephone number is (212) 845-3212.
121
SHARES ELIGIBLE FOR FUTURE SALE
Prior to this offering, there has been no public market for our common stock. Market sales of shares or the availability of shares for sale may decrease the market price of our common stock prevailing from time to time. As described below, only a portion of our outstanding shares of common stock will be available for sale shortly after this offering due to contractual and legal restrictions to resale. Nevertheless, sales of substantial amounts of common stock in the public market after these restrictions lapse, or the perception that such sales could occur, adversely affect the market price of the common stock and impair our future ability to raise capital through the sale of our equity securities.
Upon completion of this offering, 10,129,405 shares of common stock will be outstanding, assuming an initial public offering price of $10.00 per share, which is the mid-point of the range listed on the cover of this prospectus. All of the shares sold in this offering will be freely tradable. Except as set forth below, the remaining shares of common stock outstanding after this offering will be restricted as a result of securities laws or lock-up agreements. These remaining shares will be available for sale in the public market roughly as follows:
| Date of Availability of Sales |
Approximate Cumulative Number of Shares | |
| As of the date of this prospectus |
1,966,000 | |
| 90 days after the date of this prospectus |
2,474,000 | |
| 180 days after the date of this prospectus, not subject to volume limitations pursuant to Rule 144 |
7,045,000 | |
| 180 days after the date of this prospectus, subject to volume limitations pursuant to Rule 144 |
7,629,000 |
Rule 144
Under Rule 144, any non-affiliate, who has not been an affiliate of ours during the preceding three months and has held their securities for at least one year, including the holding period of any prior owner other than one of our affiliates, would be entitled to sell an unlimited number of shares immediately upon the closing of this offering without regard to whether current public information about us is available. In addition, Rule 144 provides that once we have been subject to the reporting requirements of the Exchange Act for a period of at least 90 days, non-affiliates that have held restricted securities of a reporting company for at least six months and have not had an affiliate relationship with us during the preceding three months may sell their securities without restriction or limitation, other than that Rule 144’s public information requirements must be satisfied. Rule 144 does not permit affiliates to sell restricted securities until we have been subject to the reporting requirements of the Exchange Act for a period of 90 days. After such 90 day period, Rule 144 permits affiliates that have held restricted securities for at least six months to sell such restricted securities in accordance with the traditional conditions of Rule 144, including the current public information requirement, the volume limitations, manner of sale provisions and notice requirements. In particular, an affiliate who has beneficially owned shares of our common stock for at least six months would be entitled to sell within any three-month period a number of shares that does not exceed the greater of:
| • | 1% of the number of shares of our common stock then outstanding, which will equal approximately shares immediately after this offering; or |
| • | the average weekly trading volume of our common stock on the Nasdaq Capital Market during the four calendar weeks preceding the filing of a notice on Form 144 with respect to the sale. |
Lock-Up Agreements
Upon completion of this offering, each of our officers and directors and certain of our stockholders will have agreed, subject to specified exceptions, that, without the prior written consent of the underwriters, they will
122
not, directly or indirectly, sell, offer, contract to sell, transfer the economic risk of ownership in, make any short sale, pledge or otherwise dispose of any shares of our capital stock or any securities convertible into or exchangeable or exercisable for or any other rights to purchase or acquire our capital stock for a period of 180 days from the date of this prospectus. The underwriters may, acting jointly and in their discretion, permit early release of shares subject to the lock-up agreements. See “Underwriting—Lock-ups” for a more detailed discussion of the lock-up agreements.
Registration Rights
Upon completion of this offering, the holders of the registrable shares, or their transferees, will be entitled to registration rights with respect to the registrable shares under the Securities Act. Registration of the registrable shares under the Securities Act would result in the shares becoming freely tradable without restriction under the Securities Act, except for shares purchased by affiliates, immediately upon the effectiveness of this registration. See “Description of Capital Stock—Registration Rights”.
Stock Options
We intend to file with the SEC a registration statement under the Securities Act covering the shares of common stock reserved for issuance under our stock option plans. The registration statement is expected to be filed and become effective in connection with this offering. Accordingly, shares registered under the registration statement will, subject to Rule 144 volume limitations applicable to affiliates and the lock-up agreements described above, be available for sale in the open market.
123
MATERIAL UNITED STATES FEDERAL INCOME TAX CONSIDERATIONS
FOR NON-UNITED STATES HOLDERS OF COMMON STOCK
The following is a summary of some United States federal income and estate tax consequences of the acquisition, ownership and disposition of shares of our common stock purchased pursuant to this offering by a holder that, for United States federal income tax purposes, is not a “United States person,” as we define that term below. A beneficial owner of our common stock who is not a United States person is referred to below as a “non-United States holder.” This summary is based upon current provisions of the Internal Revenue Code of 1986, as amended, Treasury regulations promulgated thereunder, judicial opinions, administrative pronouncements and published rulings of the United States Internal Revenue Service, or IRS, all as in effect as of the date hereof. These authorities may be changed, possibly retroactively, resulting in United States federal tax consequences different from those set forth below. We have not sought, and will not seek, any ruling from the IRS or opinion of counsel with respect to the statements made in the following summary, and there can be no complete assurance that the IRS will not take a position contrary to such statements or that any such contrary position taken by the IRS would not be sustained.
This summary is limited to non-United States holders who purchase shares of our common stock issued pursuant to this offering and who hold our common stock as a capital asset for United States federal income tax purposes. This summary also does not address the tax considerations arising under the laws of any state, local or non-United States jurisdiction, or under United States federal estate or gift tax laws, except as specifically described below. In addition, this summary does not address tax considerations that may be applicable to an investor’s particular circumstances nor does it address the special tax rules applicable to special classes of non-United States holders, including, without limitation:
| • | banks, insurance companies or other financial institutions; |
| • | partnerships or other entities treated as partnerships for United States federal income tax purposes; |
| • | United States expatriates; |
| • | tax-exempt organizations; |
| • | tax-qualified retirement plans; |
| • | brokers or dealers in securities or currencies; |
| • | traders in securities that elect to use a mark-to-market method of accounting for their securities holdings; or |
| • | persons that will hold common stock as a position in a hedging transaction, “straddle,” “conversion,” or other integrated transaction for tax purposes. |
If a partnership, including any entity treated as a partnership for United States federal income tax purposes, is a holder, the tax treatment of a partner in the partnership will generally depend upon the status of the partner and the activities of the partnership. A holder that is a partnership, and partners in such partnership, should consult their own tax advisors regarding the tax consequences of the purchase, ownership and disposition of shares of our common stock.
For purposes of this discussion, a United States person means a person who is for United States federal income tax purposes:
| • | a citizen or resident of the United States; |
| • | a corporation, including any entity treated as a corporation for United States federal income tax purposes created or organized under the laws of the United States, any state within the United States, or the District of Columbia; |
| • | an estate the income of which is subject to United States federal income taxation regardless of its source; or |
124
| • | a trust, if its administration is subject to the primary supervision of a United States court and one or more United States persons have the authority to control all of its substantial decisions, or other trusts considered United States persons for United States federal income tax purposes. |
YOU ARE URGED TO CONSULT YOUR TAX ADVISOR WITH RESPECT TO THE APPLICATION OF THE UNITED STATES FEDERAL INCOME TAX LAWS TO YOUR PARTICULAR SITUATION AS WELL AS ANY TAX CONSEQUENCES ARISING UNDER THE FEDERAL ESTATE OR GIFT TAX RULES OR UNDER THE LAWS OF ANY STATE, LOCAL, NON-UNITED STATES OR OTHER TAXING JURISDICTION OR UNDER ANY APPLICABLE TAX TREATY.
Dividends
If distributions are paid on shares of our common stock, the distributions will constitute dividends for United States federal income tax purposes to the extent paid from our current or accumulated earnings and profits, as determined under United States federal income tax principles. To the extent a distribution exceeds our current and accumulated earnings and profits, it will constitute a return of capital that is applied against and reduces, but not below zero, the adjusted tax basis of your shares in our common stock. Any remainder will constitute gain on the common stock. Dividends paid to a non-United States holder generally will be subject to withholding of United States federal income tax at the rate of 30% or such lower rate as may be specified by an applicable income tax treaty, the benefits of which a non-United States holder is eligible. If the dividend is effectively connected with the non-United States holder’s conduct of a trade or business in the United States or, if a tax treaty requires, attributable to a United States permanent establishment maintained by such non-United States holder, the dividend will not be subject to any withholding tax, provided certification requirements are met, as described below, but will be subject to United States federal income tax imposed on net income on the same basis that applies to United States persons generally. A corporate holder under certain circumstances also may be subject to a branch profits tax equal to 30%, or such lower rate as may be specified by an applicable income tax treaty, the benefits of which a non-United States holder is eligible, on a portion of its effectively connected earnings and profits for the taxable year. Non-United States holders should consult their own tax advisors regarding the potential applicability of any income tax treaty.
To claim the benefit of a tax treaty or to claim exemption from withholding because the income is effectively connected with the conduct of a trade or business in the United States, a non-United States holder must provide a properly executed IRS Form W-8BEN for treaty benefits or W-8ECI for effectively connected income, or such successor forms as the IRS designates, prior to the payment of dividends. These forms must be periodically updated. Non-United States holders may obtain a refund of any excess amounts withheld by timely filing an appropriate claim for refund.
Gain on Disposition
A non-United States holder generally will not be subject to United States federal income tax, including by way of withholding, on gain recognized on a sale or other disposition of shares of our common stock unless any one of the following is true:
| • | the gain is effectively connected with the non-United States holder’s conduct of a trade or business in the United States or, if a tax treaty applies, attributable to a United States permanent establishment or a fixed base maintained by such non-United States holder; |
| • | the non-United States holder is a nonresident alien individual present in the United States for 183 days or more in the taxable year of the disposition and certain other requirements are met; or |
| • | our common stock constitutes a United States real property interest by reason of our status as a “United States real property holding corporation,” or USRPHC, for United States federal income tax purposes at any time during the shorter of (1) the period during which you hold our common stock or (2) the five-year period ending on the date you dispose of our common stock. |
125
We believe that we are not currently, and will not become, a USRPHC. However, because the determination of whether we are a USRPHC depends on the fair market value of our United States real property interests relative to the fair market value of our other business assets, we cannot assure you that we will not become a USRPHC in the future. As a general matter, as long as our common stock is regularly traded on an established securities market, however, it will not be treated as a United States real property interest with respect to any non-United States holder that holds no more than 5% of such regularly traded common stock. If we are determined to be a USRPHC and the foregoing exception does not apply, among other things, a purchaser may be required to withhold 10% of the proceeds payable to a non-United States holder from a disposition of our common stock, and the non-United States holder generally will be taxed on its net gain derived from the disposition at the graduated United States federal income tax rates applicable to United States persons.
Unless an applicable treaty provides otherwise, gain described in the first bullet point above will be subject to the United States federal income tax imposed on net income on the same basis that applies to United States persons generally but will generally not be subject to withholding. Corporate holders also may be subject to a branch profits tax on such gain. Gain described in the second bullet point above will be subject to a flat 30% United States federal income tax, which may be offset by certain United States source capital losses. Non-United States holders should consult any potentially applicable income tax treaties that may provide for different rules.
United States Federal Estate Taxes
Shares of our common stock owned or treated as owned by an individual who at the time of death is a non-United States holder are considered United States situs assets and will be included in the individual’s estate for United States federal estate tax purposes, unless an applicable estate tax treaty provides otherwise.
Information Reporting and Backup Withholding
Information reporting and backup withholding (currently at a 28% rate of tax) may apply to dividends paid with respect to our common stock and to proceeds from the sale or other disposition of our common stock. If we cannot determine whether a distribution will qualify as a dividend, in whole or in part, at the time the distribution is made, then the distribution will be subject to backup withholding. In certain circumstances, non-United States holders may avoid information reporting and backup withholding if they certify under penalties of perjury as to their status as non-United States holders or otherwise establish an exemption and certain other requirements are met. Non-United States holders should consult their own tax advisors regarding the application of the information reporting and backup withholding rules to them.
Backup withholding is not an additional tax. Amounts withheld under the backup withholding rules from a payment to a non-United States holder can be refunded or credited against the non-United States holder’s United States federal income tax liability, if any, provided that an appropriate claim is timely filed with the IRS.
126
Canaccord Genuity Inc., or Canaccord Genuity, and Rodman & Renshaw, LLC, or Rodman, or collectively, the underwriters, are acting as the joint book running underwriters of this offering. Under the terms and subject to the conditions contained in an underwriting agreement dated the date of this prospectus, the underwriters have agreed to purchase, and we have agreed to sell to them, all shares of common stock offered by this prospectus as set forth below:
| Underwriter |
Shares | |
| Canaccord Genuity Inc. |
||
| Rodman & Renshaw, LLC |
Nature of Underwriting Commitment
The underwriters are offering the shares of common stock subject to its acceptance of the shares from us and subject to prior sale. The underwriting agreement provides that the obligations of the underwriters to pay for and accept delivery of the shares of common stock offered by this prospectus are subject to the approval of certain legal matters by their counsel and to other conditions. The underwriters are obligated to take and pay for all of the shares of common stock offered by this prospectus if any such shares are taken. However, the underwriters are not required to take or pay for the shares covered by the over-allotment option described below, unless and until the option is exercised. The underwriters initially propose to offer part of the shares of common stock directly to the public at the initial public offering price listed on the cover of this prospectus, less underwriting discounts and commissions, and part to certain dealers at a price that represents a concession not in excess of $ a share under the initial public offering price. The underwriters may allow, and the dealers may reallow, a discount not in excess of $ per share to other dealers. After the initial offering, the public offering price or any other term of the offering may be changed.
Option to Purchase Additional Shares
We have granted to the underwriters an option, exercisable for 30 days from the date of this prospectus, to purchase up to an aggregate of 375,000 additional shares of common stock at the initial public offering price, less underwriting discounts and commissions. The underwriters may exercise this option, in whole or in part, solely for the purpose of covering over-allotments, if any, made in connection with the offering of the shares of common stock offered by this prospectus. If the over-allotment option is exercised in full, the total price to the public would be $ , the total underwriter discounts and commissions would be $ and the total proceeds to us would be $ .
Discounts and Commissions
The following table shows the per share and total underwriting discounts and commissions that we are to pay to the underwriters in connection with this offering. These amounts are shown assuming both no exercise and full exercise of the over-allotment option.
| Total | |||||||||
| Per Share |
No Exercise |
Full Exercise | |||||||
| Public offering price |
$ | $ | $ | ||||||
| Underwriting discount |
$ | $ | $ | ||||||
| Non-accountable expense allowance |
$ | $ | $ | ||||||
| Proceeds, before expenses, to us |
$ | $ | $ | ||||||
In addition, we estimate that the expenses of this offering other than underwriting discounts and commissions payable by us will be approximately $1,930,000.
127
We have agreed to pay the underwriters a non-accountable expense allowance equal to $250,000, which is 1% of the proceeds of the offering based on the mid-point of the range listed on the cover of this prospectus, excluding any exercise of the underwriters’ over-allotment option on which the underwriters will not receive such an expense allowance. In addition, we have agreed to reimburse the underwriters for up to $60,000 of specified accountable expenses incurred in connection with this offering. We have also agreed to issue to the underwriters common stock purchase warrants to purchase up to 125,000 shares of our common stock. The warrants will have an exercise price equal to $12.50 per share assuming an initial public offering price of $10.00 of per share, which is the mid-point of range set forth on the cover of this prospectus . The warrants are exercisable commencing one (1) year after the effective date of the registration statement of which the prospectus forms a part, and will be exercisable for four (4) years thereafter. The warrants also provides for unlimited “piggyback” registration rights at our expense with respect to the underlying shares of common stock. Pursuant to the rules of the Financial Industry Regulatory, Inc., or FINRA, and in particular Rule 5110, the warrants (and underlying shares) issued to the underwriters may not be sold, transferred, assigned, pledged, or hypothecated, or the subject of any hedging, short sale, derivative, put or call transaction that would result in the effective disposition of the securities by any person for a period of one year immediately following the date of delivery and payment for the shares offered; provided, however, that the warrants (and underlying shares) may be transferred to officers or partners of the underwriters as long as the warrants (and underlying shares) remain subject to the lockup. In the event that the offering is not consummated following the execution of the underwriting agreement, we have agreed to reimburse the actual and accountable legal expenses of the underwriters up to a maximum of $250,000.
Lock-ups
Upon completion of this offering, each of our officers and directors and certain stockholders will have agreed that, subject to specified exceptions, without the prior written consent of the underwriters acting jointly through their representative Canaccord Genuity, they will not, during the period beginning on the date of this prospectus and ending 180 days thereafter:
| • | offer, pledge, sell, announce the intention to sell, contract to sell, sell any option or contract to purchase, purchase any option or contract to sell, grant any option, right or warrant to purchase, lend, or otherwise transfer or dispose of, directly or indirectly, any shares of common stock or any securities convertible into or exercisable or exchangeable for common stock; |
| • | enter into any swap or other arrangement that transfers to another, in whole or in part, any of the economic consequences of ownership of common stock; or |
| • | make any demand for or exercise any right with respect to, the registration of any shares of common stock or any security convertible into or exercisable or exchangeable for common stock; |
whether any such transaction described above is to be settled by delivery of common stock or such other securities, in cash or otherwise.
The restrictions described in the preceding paragraphs do not apply to:
| • | the sale by us of shares to the underwriter in connection with the offering; |
| • | options issued pursuant to employee benefit plans; |
| • | transactions by any person other than us relating to shares of common stock or other securities convertible or exchangeable into common stock acquired in open market transactions after the completion of the offering of the shares; or |
| • | the transfer of shares of common stock or any security convertible or exchangeable into shares of common stock as a bona fide gift, as a distribution to general or limited partners, stockholders, members or affiliates of our stockholders, or by will or intestate succession to a member of the immediate family of our stockholders, or to a trust for the benefit of such immediate family member. |
With respect to the last bullet, it shall be a condition to the transfer or distribution that the donor or transferor provide prior written notice of such transfer or distribution to Canaccord Genuity, the donee or transferee execute a copy of the lock-up agreement, no filing by any donee or transferee with the SEC shall be required or shall be made voluntarily in connection with such transfer or distribution, other than a filing on Form 5, and no such transfer or distribution may include a disposition for value.
128
The 180-day restricted period described in the preceding paragraph will be extended if:
| • | during the last 17 days of the 180-day restricted period we issue an earnings release or material news or a material event relating to us occurs; or |
| • | prior to the expiration of the 180-day restricted period, we announce that we will release earnings results during the 16-day period beginning on the last day of the 180-day restricted period; |
in which case the restrictions described in the preceding paragraph will continue to apply until the expiration of the 18-day period beginning on the issuance of the earnings release or the occurrence of the material news or material event.
Stabilization
In order to facilitate this offering of common stock, the underwriters may engage in transactions that stabilize, maintain or otherwise affect the price of the common stock. Specifically, the underwriters may sell more shares than they are obligated to purchase under the underwriting agreement, creating a short position. A short sale is covered if the short position is no greater than the number of shares available for purchase by the underwriters under the over-allotment option. The underwriters can close out a covered short sale by exercising the over- allotment option or by purchasing shares in the open market. In determining the source of shares to close out a covered short sale, the underwriters will consider, among other things, the open market price of shares compared to the price available under the over-allotment option. The underwriters may also sell shares in excess of the over-allotment option, creating a naked short position. The underwriters must close out any naked short position by purchasing shares in the open market. A naked short position is more likely to be created if the underwriters are concerned that there may be downward pressure on the price of the common stock in the open market after pricing that could adversely affect investors who purchase in this offering. In addition, to stabilize the price of the common stock, the underwriters may bid for and purchase shares of common stock in the open market. Finally, the underwriters may reclaim selling concessions allowed for distributing the common stock in the offering if the syndicate repurchases previously distributed common stock to cover syndicate short positions or to stabilize the price of the common stock. These activities may raise or maintain the market price of the common stock above independent market levels or prevent or retard a decline in the market price of the common stock. The underwriters are not required to engage in these activities and may end any of these activities at any time.
Other Terms
We have been approved to have our common stock quoted on the Nasdaq Capital Market under the symbol “SRGV.”
We and the underwriters have agreed to indemnify each other against certain liabilities, including liabilities under the Securities Act.
Prior to this offering, there has been no public market for the shares of common stock. The initial public offering price will be determined by negotiations between us and the underwriters. Among the factors to be considered in determining the initial public offering price will be our future prospects and those of our industry in general; sales, earnings and other financial operating information in recent periods; and the price-earnings ratios, price-sales ratios and market prices of securities and certain financial and operating information of companies engaged in activities similar to ours. The estimated initial public offering price range set forth on the cover of this prospectus is subject to change as a result of market conditions and other factors. An active trading market for the shares may not develop, and it is possible that after the offering the shares will not trade in the market above their initial offering price. A prospectus in electronic format may be made available on a web site maintained by the underwriters, and the underwriters may distribute prospectuses electronically.
129
Foreign Regulatory Restrictions on Purchase of Our Common Stock
We have not taken any action to permit a public offering of our common stock outside the United States or to permit the possession or distribution of this prospectus outside the United States. Persons outside the United States who come into possession of this prospectus must inform themselves about and observe any restrictions relating to this offering and the distribution of the prospectus outside the United States. In addition to the public offering of our common stock in the United States, the underwriters may, subject to applicable foreign laws, also offer our common stock to certain institutions or accredited persons in other countries.
Italy
The offering of shares of common stock pursuant to this prospectus has not been cleared by Consob, the Italian Stock Exchange’s regulatory agency of public companies, pursuant to Italian securities legislation and, accordingly, no shares may be offered, sold or delivered, nor may copies of this prospectus or of any other document relating to our common stock be distributed in Italy, except (1) to professional investors (operatori qualificati); or (2) in circumstances which are exempted from the rules on solicitation of investments pursuant to Decree No. 58 and Article 33, first paragraph, of Consob Regulation No. 11971 of May 14, 1999, as amended. Any offer, sale or delivery of our common stock or distribution of copies of this prospectus or any other document relating to our common stock in Italy under (1) or (2) above must be (i) made by an investment firm, bank or financial intermediary permitted to conduct such activities in Italy in accordance with the Decree No. 58 and Legislative Decree No. 385 of September 1, 1993, or the Banking Act; and (ii) in compliance with Article 129 of the Banking Act and the implementing guidelines of the Bank of Italy, as amended from time to time, pursuant to which the issue or the offer of securities in Italy may need to be preceded and followed by an appropriate notice to be filed with the Bank of Italy depending, inter alia, on the aggregate value of the securities issued or offered in Italy and their characteristics; and (iii) in compliance with any other applicable laws and regulations.
Germany
The offering of our common stock is not a public offering in the Federal Republic of Germany. The shares may only be acquired in accordance with the provisions of the Securities Sales Prospectus Act (Wertpapier-Verkaudfspropsektgestz), as amended, and any other applicable German law. No application has been made under German law to publicly market our common stock in or out of the Federal Republic of Germany. Our common stock is not registered or authorized for distribution under the Securities Sales Prospectus Act and accordingly may not be, and are not being, offered or advertised publicly or by public promotion. This prospectus is strictly for private use and the offering is only being made to recipients to whom the document is personally addressed and does not constitute an offer or advertisement to the public. Our common stock will only be available to persons who, by profession, trade or business, buy or sell securities for their own or a third party’s account.
France
Our common stock offered by this prospectus may not be offered or sold, directly or indirectly, to the public in France. This prospectus has not been, and will not be, submitted to the clearance procedure of the Autorité des Marchés Financiers, or the AMF, and may not be released or distributed to the public in France. Investors in France may only purchase the common stock offered by this prospectus for their own account and in accordance with articles L. 411-1, L. 441-2 and L. 412-1 of the Code Monétaire et Financier and decree no. 98-880 dated October 1, 1998, provided they are “qualified investors” within the meaning of said decree. Each French investor must represent in writing that it is a qualified investor within the meaning of the aforesaid decree. Any resale, directly or indirectly, to the public of the common stock offered by this prospectus may be effected only in compliance with the above mentioned regulations. “Les actions offertes par ce document d’information ne peuvent pas être, directement ou indirectement, offertes ou vendues au public en France. Ce document d’information n’a pas été ou ne sera pas soumis au visa de l’Autorité des Marchés Financiers et ne peut être diffusé ou distribué au public en France. Les investisseurs en France ne peuvent acheter les actions offertes par ce document d’information que pour leur compte propre et conformément aux articles L. 411-1, L. 441-2 et
130
L. 412-1 du Code Monétaire et Financier et du décret no. 98-880 du 1 octobre 1998, sous réserve qu’ils soient des investisseurs qualifiés au sens du décret susvisé. Chaque investisseur doit déclarer par écrit qu’il est un investisseur qualifié au sens du décret susvisé. Toute revente, directe ou indirecte, des actions offertes par ce document d’information au public ne peut être effectuée que conformément à la réglementation susmentionnée.”
Greece
This prospectus has been submitted for approval by the SEC and not the Greek Capital Market Committee. All information contained in this prospectus is true and accurate. The offering of our common stock does not constitute an initial public offering in Greece according to CL. 2190/1920 and L. 3401/2005 as amended and in force. This prospectus is strictly for the use of the person or entity to which it has been addressed to by us and not to be circulated in Greece or any other jurisdiction.
This information and documentation is true and accurate and in conformity with the information contained in the prospectus for the offer of common stock, which is being reviewed for approval only by the SEC, and does not constitute provision of the investment service of investment advice according to L. 3606/2007. Any recipient of this material has stated to be a qualified and experienced investor and will evaluate the contents and decide on his/her own discretion whether to participate or not in the offering pursuant to this prospectus.
United Kingdom
In the United Kingdom, the shares of common stock offered by this prospectus are directed to and will only be available for purchase to a person who is an exempt person in accordance with clause (c) below and who warrants, represents and agrees that: (a) it has not offered or sold, will not offer or sell, any shares offered by this prospectus to any person in the United Kingdom except in circumstances that do not constitute an offer to the public in the United Kingdom for the purposes of the section 85 of the Financial Services and Markets Act 2000 (as amended), or the FSMA; and (b) it has complied and will comply with all applicable provisions of FSMA and the regulations made thereunder in respect of anything done by it in relation to the common stock offered by this prospectus in, from or otherwise involving the United Kingdom; and (c) it is a person who falls within the exemptions to Section 21 of the FSMA as set out in The Financial Services and Markets Act 2000 (Financial Promotion) Order 2005, or the Order, being either an investment professional as described under Article 19 or any body corporate (which itself has or a group undertaking has a called up share capital or net assets of not less than £500,000 (if more than 20 members) or otherwise £5 million) or an unincorporated association or partnership (with net assets of not less than £5 million) or is a trustee of a high value trust or any person acting in the capacity of director, officer or employee of such entities as defined under Article 49(2)(a) to (d) of the Order, or a person to whom the invitation or inducement may otherwise lawfully be communicated or cause to be communicated. The investment activity to which this document relates will only be available to and engaged in only with exempt persons referred to above. Persons who are not investment professionals and do not have professional experience in matters relating to investments or are not an exempt person as described above, should not review nor rely or act upon this document and should return this document immediately. It should be noted that this document is not a prospectus in the United Kingdom as defined in the Prospectus Regulations 2005 and has not been approved by the Financial Services Authority or any competent authority in the United Kingdom.
Sweden
Neither this prospectus nor the common stock offered hereunder has been registered with or approved by the Swedish Financial Supervisory Authority under the Swedish Financial Instruments Trading Act (1991:980) (as amended), nor will such registration or approval be sought. Accordingly, this prospectus may not be made available nor may the shares of common stock offered hereunder be marketed or offered for sale in Sweden other than in circumstances that are deemed not to be an offer to the public in Sweden under the Financial Instruments Trading Act. This prospectus may not be distributed to the public in Sweden and a Swedish recipient of this prospectus may not in any way forward this prospectus to the public in Sweden.
131
Norway
This prospectus has not been produced in accordance with the prospectus requirements laid down in the Norwegian Securities Trading Act 1997, as amended. This prospectus has not been approved or disapproved by, or registered with, either the Oslo Stock Exchange or the Norwegian Registry of Business Enterprises. This prospectus may not, either directly or indirectly, be distributed to Norwegian potential investors.
Denmark
This prospectus has not been prepared in the context of a public offering of securities in Denmark within the meaning of the Danish Securities Trading Act No. 171 of 17 March 2005, as amended from time to time, or any Executive Orders issued on the basis thereof and has not been and will not be filed with or approved by the Danish Financial Supervisory Authority or any other public authority in Denmark. The offering of the shares of common stock pursuant to this prospectus will only be made to persons pursuant to one or more of the exemptions set out in Executive Order No. 306 of 28 April 2005 on Prospectuses for Securities Admitted for Listing or Trade on a Regulated Market and on the First Public Offer of Securities exceeding EUR 2,500,000 or Executive Order No. 307 of 28 April 2005 on Prospectuses for the First Public Offer of Certain Securities between EUR 100,000 and EUR 2,500,000, as applicable.
The Netherlands
The underwriters may not offer, distribute, sell, transfer or deliver any of our securities, directly or indirectly, in The Netherlands, as a part of their initial distribution or at any time thereafter, to any person other than our employees or employees of our subsidiary, individuals who or legal entities which trade or invest in securities in the conduct of their profession or business within the meaning of article 2 of the Exemption Regulation issued under the Securities Transactions Supervision Act 1995 (Vrijstellingsregeling Wet toezich teffectenverkeer1995), which includes banks, brokers, pension funds, insurance companies, securities institutions, investment institutions, and other institutional investors, including, among others, treasuries of large enterprises who or which regularly trade or invest in securities in a professional capacity.
Cyprus
The underwriters have represented, warranted and agreed that: (i) they will not be providing from or within Cyprus any “Investment Services,” “Investment Activities” and “Non-Core Services” (as such terms are defined in the Investment Firms Law 144(I) of 2007, or the IFL) in relation to the shares of common stock, or will be otherwise providing Investment Services, Investment Activities and Non-Core Services to residents or persons domiciled in Cyprus. The underwriters have represented, warranted and agreed that it will not be concluding in Cyprus any transaction relating to such Investment Services, Investment Activities and Non-Core Services in contravention of the IFL and/or applicable regulations adopted pursuant thereto or in relation thereto; and (ii) they have not and will not offer any of the common stock other than in compliance with the provisions of the Public Offer and Prospectus Law, Law 114(I)/2005.
Switzerland
This prospectus may only be used by those persons to whom it has been directly handed out by the offeror or its designated distributors in connection with the offer described therein. The common stock is only offered to those persons and/or entities directly solicited by the offeror or its designated distributors, and are not offered to the public in Switzerland. This prospectus constitutes neither a public offer in Switzerland nor an issue prospectus in accordance with the respective Swiss legislation, in particular but not limited to Article 652A Swiss Code Obligations. Accordingly, this prospectus may not be used in connection with any other offer, whether private or public and shall in particular not be distributed to the public in Switzerland.
132
Israel
The common stock offered by this prospectus has not been approved or disapproved by the Israeli Securities Authority, or ISA. The shares may not be offered or sold, directly or indirectly, to the public in Israel. The ISA has not issued permits, approvals or licenses in connection with the offering or publishing the prospectus; nor has it authenticated the details included herein, confirmed their reliability or completeness, or rendered an opinion as to the quality of the common stock being offered. Any resale, directly or indirectly, to the public of the common stock offered by this prospectus is subject to restrictions on transferability and must be effected only in compliance with the Israeli securities laws and regulations.
Oman
For the attention of the residents of Oman:
The information contained in this prospectus neither constitutes a public offer of securities in the Sultanate of Oman, or Oman, as contemplated by the Commercial Companies Law of Oman (Sultani Decree 4/74) or the Capital Market Law of Oman (Sultani Decree 80/98), nor does it constitute an offer to sell, or the solicitation of any offer to buy non-Omani securities in Oman as contemplated by Article 6 of the Executive Regulations to the Capital Market Law of Oman (issued vide Ministerial Decision No 4/2001), and nor does it constitute a distribution of non-Omani securities in Oman as contemplated under the Rules for Distribution of Non-Omani Securities in Oman issued by the Capital Market Authority of Oman, or CMA. Additionally, this prospectus is not intended to lead to the conclusion of any contract of whatsoever nature within the territory of Oman.
This prospectus has been sent at the request of the investor in Oman, and by receiving this prospectus, the person or entity to whom it has been issued and sent understands, acknowledges and agrees that this prospectus has not been approved by the CMA or any other regulatory body or authority in Oman, nor has any authorization, license or approval been received from the CMA or any other regulatory authority in Oman, to market, offer, sell, or distribute the shares within Oman.
No marketing, offering, selling or distribution of any financial or investment products or services has been or will be made from within Oman and no subscription to any securities, products or financial services may or will be consummated within Oman. Neither of the underwriters are a company licensed by the CMA to provide investment advisory, brokerage, or portfolio management services in Oman, nor a bank licensed by the Central Bank of Oman to provide investment banking services in Oman. Neither of the underwriters advise persons or entities resident or based in Oman as to the appropriateness of investing in or purchasing or selling securities or other financial products.
Nothing contained in this prospectus is intended to constitute Omani investment, legal, tax, accounting or other professional advice. This prospectus is for your information only, and nothing herein is intended to endorse or recommend a particular course of action. You should consult with an appropriate professional for specific advice on the basis of your situation.
United Arab Emirates
This document has not been reviewed, approved or licensed by the Central Bank of the United Arab Emirates, or the UAE, Emirates Securities and Commodities Authority or any other relevant licensing authority in the UAE including any licensing authority incorporated under the laws and regulations of any of the free zones established and operating in the territory of the UAE, in particular the Dubai International Financial Services Authority, or the DFSA, a regulatory authority of the Dubai International Financial Centre, or the DIFC. The sale of the shares does not constitute a public offer of securities in the UAE, DIFC and/or any other free zone in accordance with the Commercial Companies Law, Federal Law No. 8 of 1984 (as amended), DFSA Offered Securities Rules and the Dubai International Financial Exchange Listing Rules, accordingly, or otherwise.
133
The shares may not be offered to the public in the UAE and/or any of the free zones including, in particular, the DIFC. The shares may be offered and this document may be issued, only to a limited number of investors in the UAE or any of its free zones (including, in particular, the DIFC) who qualify as sophisticated investors under the relevant laws and regulations of the UAE or the free zone concerned. Our management and the underwriters represent and warrant that the shares will not be offered, sold, transferred or delivered to the public in the UAE or any of its free zones including, in particular, the DIFC.
People’s Republic of China
This prospectus may not be circulated or distributed in the People’s Republic of China, or PRC, and our common stock may not be offered or sold to any person for re-offering or resale, directly or indirectly, to any resident of the PRC except pursuant to applicable laws and regulations of the PRC. For the purpose of this paragraph, PRC does not include Taiwan and the special administrative regions of Hong Kong and Macau.
Botswana
We hereby represent and warrant that we have not offered for sale or sold, and will not offer or sell, directly or indirectly our common stock to the public in the Republic of Botswana, and confirms that the offering will not be subject to any registration requirements as a prospectus pursuant to the requirements and/or provisions of the Companies Act, 2003 or the Listing Requirements of the Botswana Stock Exchange.
Hong Kong
The shares of common stock have not been offered or sold and will not be offered or sold in Hong Kong, by means of any document, other than (a) to “professional investors” as defined in the Securities and Futures Ordinance (Cap. 571) of Hong Kong and any rules made under that ordinance; or (b) in other circumstances which do not result in the document being a “prospectus” as defined in the Companies ordinance (Cap. 32) of Hong Kong or which do not constitute an offer to the public within the meaning of that ordinance. No advertisement, invitation or document, whether in Hong Kong or elsewhere, which is directed at, or the contents of which are likely to be accessed or read by, the public of Hong Kong (except if permitted to do so under the securities laws of Hong Kong) has been issued or will be issued in Hong Kong or elsewhere other than with respect to the shares of common stock that are or are intended to be disposed of only to persons outside Hong Kong or only to “professional investors” within the meaning of the Securities and Futures Ordinance (Cap. 571) of Hong Kong and any rules made under that ordinance.
The contents of this document have not been reviewed by any regulatory authority in Hong Kong. You are advised to exercise caution in relation to the offer. If you are in any doubt about any of the contents of this document, you should obtain independent professional advice.
Singapore
This prospectus has not been registered as a prospectus with the Monetary Authority of Singapore. Accordingly, this prospectus and any other document or material in connection with the offer or sale, or invitation for subscription or purchase, of the shares of common stock may not be circulated or distributed, nor may the shares of common stock be offered or sold, or be made the subject of an invitation for subscription or purchase, whether directly or indirectly, to persons in Singapore other than (i) to an institutional investor under Section 274 of the Securities and Futures Act, Chapter 289 of Singapore, or the SFA, (ii) to a relevant person, or any person pursuant to Section 275(1A), and in accordance with the conditions, specified in Section 275 of the SFA or (iii) otherwise pursuant to, and in accordance with the conditions of, any other applicable provision of the SFA.
134
Where the shares of common stock are subscribed or purchased under Section 275 by a relevant person which is: (a) a corporation (which is not an accredited investor) the sole business of which is to hold investments and the entire share capital of which is owned by one or more individuals, each of whom is an accredited investor; or (b) a trust (where the trustee is not an accredited investor) whose sole purpose is to hold investments and each beneficiary is an accredited investor, shares, debentures and units of shares and debentures of that corporation or the beneficiaries’ rights and interest in that trust shall not be transferable for 6 months after that corporation or that trust has acquired the shares under Section 275 except: (1) to an institutional investor under Section 274 of the SFA or to a relevant person, or any person pursuant to Section 275(1A), and in accordance with the conditions, specified in Section 275 of the SFA; (2) where no consideration is given for the transfer; or (3) by operation of law.
Canada
NOTICE TO CANADIAN INVESTORS
Resale Restrictions
The distribution of our common stock in Canada is being made only on a private placement basis exempt from the requirement that we prepare and file a prospectus with the applicable securities regulatory authorities. We are not a reporting issuer (or equivalent) in any province or territory in Canada and our common stock is not listed on any stock exchange in Canada and there is currently no public market for our common stock in Canada. We currently have no intention of becoming a reporting issuer in Canada, filing a prospectus with any common stock regulatory authority in Canada to qualify the resale of the common stock to the public, or listing our common stock on any stock exchange in Canada. Accordingly, to be made in accordance with securities laws, any resale of the common stock in Canada must be made under available statutory exemptions from registration and prospectus requirements or under a discretionary exemption granted by the applicable Canadian securities regulatory authority. Canadian purchasers are advised to seek legal and tax advice prior to any purchase or resale of our common stock.
European Economic Area
NOTICE TO PROSPECTIVE INVESTORS IN THE EEA
In relation to each member state of the European Economic Area, or EEA, which has implemented the Prospectus Directive (each, a “Relevant Member State”) an offer to the public of any shares of our common stock which are the subject of the offering contemplated by this prospectus may not be made in that Relevant Member State, except that an offer to the public in that Relevant Member State of any shares may be made at any time under the following exemptions under the Prospectus Directive, if they have been implemented in that Relevant Member State:
(a) to legal entities which are authorized or regulated to operate in the financial markets or, if not so authorized or regulated, whose corporate purpose is solely to invest in securities;
(b) to any legal entity which has two or more of (1) an average of at least 250 employees during the last financial year; (2) a total balance sheet of more than €43,000,000 and (3) an annual net turnover of more than €50,000,000, as shown in its last annual or consolidated accounts;
(c) by the underwriters to fewer than 100 natural or legal persons (other than “qualified investors” as defined in the Prospectus Directive) subject to obtaining the prior consent of the representatives for any such offer; or
(d) in any other circumstances falling within Article 3(2) of the Prospectus Directive; provided that no such offer of shares shall result in a requirement for the publication by us or any representative of a prospectus pursuant to Article 3 of the Prospectus Directive.
135
Any person making or intending to make any offer of shares within the EEA should only do so in circumstances in which no obligation arises for us or the underwriters to produce a prospectus for such offer. Neither we nor the underwriters have authorized, nor do they authorize, the making of any offer of shares through any financial intermediary, other than offers made by the underwriters which constitute the final offering of shares contemplated in this prospectus.
For the purposes of this provision, and your representation below, the expression of an “offer to the public” in relation to any shares in any Relevant Member State means the communication in any form and by any means of sufficient information on the terms of the offer and any shares to be offered so as to enable an investor to decide to purchase any shares, as the same may be varied in that Relevant Member State by any measure implementing the Prospectus Directive in that Relevant Member State and the expression “Prospectus Directive” means Directive 2003/71/EC and includes any relevant implementing measure in each Relevant Member State.
Each person in a Relevant Member State who receives any communication in respect of, or who acquires any shares under, the offer of shares of our common stock contemplated by this prospectus will be deemed to have represented, warranted and agreed to and with us and the underwriters that:
(A) it is a “qualified investor” within the meaning of the law in that Relevant Member State implementing Article 2(1)(e) of the Prospectus Directive; and
(B) in the case of any shares acquired by it as a financial intermediary, as that term is used in Article 3(2) of the Prospectus Directive, (i) the shares acquired by it in the offering have not been acquired on behalf of, nor have they been acquired with a view to their offer or resale to, persons in any Relevant Member State other than “qualified investors” (as defined in the Prospectus Directive), or in circumstances in which the prior consent of the representatives has been given to the offer or resale; or (ii) where shares have been acquired by it on behalf of persons in any Relevant Member State other than qualified investors, the offer of those shares to it is not treated under the Prospectus Directive as having been made to such persons.
The validity of the shares of common stock offered hereby and certain other legal matters will be passed upon for us by Baker, Donelson, Bearman, Caldwell & Berkowitz, PC, Memphis, Tennessee. Certain legal matters will be passed upon for the underwriters by Andrews Kurth LLP, Austin, Texas.
The financial statements of SurgiVision, Inc. as of December 31, 2009 and 2008 and for each of the three years in the period ended December 31, 2009 appearing in this prospectus and registration statement, have been audited by Cherry, Bekaert & Holland, L.L.P., independent registered public accounting firm, as set forth in their report thereon appearing elsewhere herein, and are included in reliance upon such report, given on the authority of such firm as experts in accounting and auditing.
136
WHERE YOU CAN FIND MORE INFORMATION
We have filed with the SEC a registration statement on Form S-1 under the Securities Act of 1933, as amended, with respect to the shares of common stock being offered by this prospectus. This prospectus does not contain all of the information in the registration statement and its exhibits. For further information with respect to SurgiVision, Inc. and the common stock offered by this prospectus, we refer you to the registration statement and its exhibits. Statements contained in this prospectus as to the contents of any contract or any other document referred to are not necessarily complete, and in each instance, we refer you to the copy of the contract or other document filed as an exhibit to the registration statement. Each of these statements is qualified in all respects by this reference.
You can read our SEC filings, including the registration statement, over the Internet at the SEC’s website at http://www.sec.gov. You may also read and copy any document we file with the SEC at its public reference facilities at 100 F Street NE, Washington, D.C. 20549. You may also obtain copies of these documents at prescribed rates by writing to the Public Reference Section of the SEC at 100 F Street NE, Washington, D.C. 20549. Please call the SEC at 1-800-SEC-0330 for further information on the operation of the public reference facilities.
Upon completion of this offering, we will be subject to the information reporting requirements of the Securities Exchange Act of 1934, as amended, and we will file reports, proxy statements and other information with the SEC. These reports, proxy statements and other information will be available for inspection and copying at the public reference room and website of the SEC referred to above. We also maintain a website at http://www.surgivision.com, at which you may access these materials free of charge as soon as reasonably practicable after they are electronically filed with, or furnished to, the SEC. The information contained in, or that can be accessed through, our website is not part of this prospectus.
137
INDEX TO FINANCIAL STATEMENTS
| Page | ||
| Report of Independent Registered Public Accounting Firm—Cherry, Bekaert & Holland, L.L.P. |
F-2 | |
| Balance Sheets as of March 31, 2010 (unaudited) and December 31, 2009 and 2008 |
F-3 | |
| F-4 | ||
| F-5 | ||
| F-6 | ||
| F-8 |
F-1
Report of Independent Registered Public Accounting Firm
To the Board of Directors and Stockholders of
SurgiVision, Inc.
We have audited the accompanying balance sheets of SurgiVision, Inc., a Delaware corporation (the “Company”), as of December 31, 2009 and 2008, and the related statements of operations, stockholders’ equity (deficit) and cash flows for the years ended December 31, 2009, 2008, and 2007. These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. Our audits included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purposes of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the accompanying financial statements present fairly, in all material respects, the financial position of SurgiVision, Inc. as of December 31, 2009 and 2008 and the results of its operations and its cash flows for the years ended December 31, 2009, 2008, and 2007 in conformity with accounting principles generally accepted in the United States of America.
The accompanying financial statements have been prepared assuming the Company will continue as a going concern. As discussed in Note 3 to the financial statements, the Company incurred net losses during the three years ended December 31, 2009 of approximately $16,287,000 and will require additional financing to fund the continued development of the Company’s products. The availability of such financing cannot be assured. These conditions raise substantial doubt about the Company’s ability to continue as a going concern. Management’s plans with regard to these matters are described in Note 3. The financial statements do not include any adjustments with respect to the possible future effects on the recoverability and classification of assets or the amounts and classification of liabilities that might result from the outcome of this uncertainty.
/s/ Cherry, Bekaert & Holland, L.L.P.
Tampa, Florida
July 21, 2010
F-2
Balance Sheets
| (unaudited) March 31, 2010 |
December 31, | |||||||||||
| 2009 | 2008 | |||||||||||
| ASSETS | ||||||||||||
| Current assets |
||||||||||||
| Cash and cash equivalents |
$ | 3,548,719 | $ | 2,569,129 | $ | 9,920,801 | ||||||
| Due from related parties |
— | 204 | 8,317 | |||||||||
| Inventory |
646,431 | 569,350 | — | |||||||||
| Prepaid expenses and other current assets |
135,626 | 54,823 | 21,440 | |||||||||
| Total current assets |
4,330,776 | 3,193,506 | 9,950,558 | |||||||||
| Furniture, software and equipment, net |
982,776 | 992,158 | 860,506 | |||||||||
| Deferred offering costs |
736,590 | 366,503 | — | |||||||||
| Deferred financing costs |
407,013 | — | — | |||||||||
| Licenses, net |
58,500 | 63,000 | 81,000 | |||||||||
| Deposits |
58,521 | 58,521 | 63,296 | |||||||||
| Total assets |
$ | 6,574,176 | $ | 4,673,688 | $ | 10,955,360 | ||||||
| LIABILITIES AND STOCKHOLDERS’ DEFICIT | ||||||||||||
| Current liabilities |
||||||||||||
| Accounts payable |
$ | 766,362 | $ | 473,484 | $ | 742,498 | ||||||
| Accrued compensation |
142,535 | 539,865 | 180,852 | |||||||||
| Other accrued liabilities |
1,383,403 | 704,000 | 111,272 | |||||||||
| Income taxes payable |
— | 49,250 | — | |||||||||
| Derivative liability |
1,436,850 | 1,227,500 | — | |||||||||
| Related party deferred revenue |
2,600,000 | 2,600,000 | 2,600,000 | |||||||||
| Total current liabilities |
6,329,150 | 5,594,099 | 3,634,622 | |||||||||
| Related party deferred revenue |
5,946,374 | 6,596,374 | 9,085,099 | |||||||||
| Related party convertible notes payable, net of unamortized discount of $1,063,270 and $1,129,000 at March 31, 2010 and December 31, 2009, respectively |
2,436,730 | 2,371,000 | — | |||||||||
| Senior unsecured convertible notes, net of unamortized discount of $814,174 |
3,256,826 | — | — | |||||||||
| Total liabilities |
17,969,080 | 14,561,473 | 12,719,721 | |||||||||
| Commitments and contingencies (Notes 2, 5, 10 and 11) |
— | — | — | |||||||||
| Stockholders’ deficit |
||||||||||||
| Series A convertible preferred stock; $.01 par value; 8,000,000 shares authorized and 7,965,000 shares issued and outstanding |
7,965,000 | 7,965,000 | 7,965,000 | |||||||||
| Common stock, $.01 par value; 70,000,000 shares authorized; 5,455,110 (2010 and 2009) and 5,451,777 (2008) issued; 5,129,280 (2010 and 2009) and 5,451,777 (2008) outstanding |
54,551 | 54,551 | 54,518 | |||||||||
| Additional paid-in capital |
26,803,455 | 25,794,862 | 25,653,645 | |||||||||
| Treasury stock, at cost, 325,830 shares |
(1,679,234 | ) | (1,679,234 | ) | — | |||||||
| Notes receivable, stockholder |
— | — | (573,620 | ) | ||||||||
| Accumulated deficit |
(44,538,676 | ) | (42,022,964 | ) | (34,863,904 | ) | ||||||
| Total stockholders’ deficit |
(11,394,904 | ) | (9,887,785 | ) | (1,764,361 | ) | ||||||
| Total liabilities and stockholders’ deficit |
$ | 6,574,176 | $ | 4,673,688 | $ | 10,955,360 | ||||||
See notes to financial statements.
F-3
Statements of Operations
| (unaudited) Three Months Ended March 31, |
Years Ended December 31, | |||||||||||||||||||
| 2010 | 2009 | 2009 | 2008 | 2007 | ||||||||||||||||
| Related party license revenue |
$ | 650,000 | $ | 650,000 | $ | 2,600,000 | $ | 1,950,000 | $ | — | ||||||||||
| Operating costs and expenses: |
||||||||||||||||||||
| Research and development costs |
1,747,395 | 1,501,555 | 6,067,617 | 4,258,492 | 2,098,672 | |||||||||||||||
| General and administrative expenses |
1,011,747 | 605,683 | 3,595,917 | 2,920,311 | 1,413,369 | |||||||||||||||
| Total operating costs and expenses |
2,759,142 | 2,107,238 | 9,663,534 | 7,178,803 | 3,512,041 | |||||||||||||||
| Loss from operations |
(2,109,142 | ) | (1,457,238 | ) | (7,063,534 | ) | (5,228,803 | ) | (3,512,041 | ) | ||||||||||
| Other income (expense): |
||||||||||||||||||||
| Loss on change in fair value of derivative liability |
(209,350 | ) | — | — | — | — | ||||||||||||||
| Interest income |
3,822 | 32,325 | 106,197 | 193,756 | 209,641 | |||||||||||||||
| Related party interest expense |
(153,424 | ) | — | (152,473 | ) | (394,738 | ) | (394,737 | ) | |||||||||||
| Other interest expense |
(47,618 | ) | — | — | — | — | ||||||||||||||
| Loss before income taxes |
(2,515,712 | ) | (1,424,913 | ) | (7,109,810 | ) | (5,429,785 | ) | (3,697,137 | ) | ||||||||||
| Income taxes |
— | — | 49,250 | — | — | |||||||||||||||
| Net loss |
$ | (2,515,712 | ) | $ | (1,424,913 | ) | $ | (7,159,060 | ) | $ | (5,429,785 | ) | $ | (3,697,137 | ) | |||||
| Net loss per share attributable to common stockholders: |
||||||||||||||||||||
| Basic and diluted |
$ | (0.49 | ) | $ | (0.27 | ) | $ | (1.34 | ) | $ | (1.04 | ) | $ | (0.74 | ) | |||||
| Weighted average shares outstanding: |
||||||||||||||||||||
| Basic |
5,129,280 | 5,368,444 | 5,336,633 | 5,245,081 | 5,024,515 | |||||||||||||||
See notes to financial statements.
F-4
Statements of Stockholders’ Equity (Deficit)
for the Years Ended December 31, 2007, 2008, and 2009 and the Three Months Ended March 31, 2010 (unaudited)
| Convertible Preferred Stock Series A |
Common Stock | Additional Paid-in Capital |
Treasury Stock |
Stock Subscription Receivable |
Notes Receivable Stockholders |
Accumulated Deficit |
Total | |||||||||||||||||||||||||||
| Shares | Amount | Shares | Amount | |||||||||||||||||||||||||||||||
| Balances, January 1, 2007 |
7,965,000 | $ | 7,965,000 | 4,969,471 | $ | 49,695 | $ | 23,172,908 | $ | — | $ | (100,000 | ) | $ | (530,361 | ) | $ | (25,736,982 | ) | $ | 4,820,260 | |||||||||||||
| Collection of stock subscription receivable |
— | — | — | — | — | — | 100,000 | — | — | 100,000 | ||||||||||||||||||||||||
| Accrued interest on note receivable |
— | — | — | — | — | — | — | (21,600 | ) | — | (21,600 | ) | ||||||||||||||||||||||
| Common stock issued in connection with research and consulting agreement |
— | — | 63,846 | 638 | 55,546 | — | — | — | — | 56,184 | ||||||||||||||||||||||||
| Common stock issued in connection with consulting agreement |
— | — | 500 | 5 | 1,595 | — | — | — | — | 1,600 | ||||||||||||||||||||||||
| Warrants issued in connection with convertible note payable amendment |
— | — | — | — | 789,475 | — | — | — | — | 789,475 | ||||||||||||||||||||||||
| Employee share-based compensation |
— | — | — | — | 20,401 | — | — | — | — | 20,401 | ||||||||||||||||||||||||
| Net loss for the year |
— | — | — | — | — | — | — | — | (3,697,137 | ) | (3,697,137 | ) | ||||||||||||||||||||||
| Balances, December 31, 2007 |
7,965,000 | 7,965,000 | 5,033,817 | 50,338 | 24,039,925 | — | — | (551,961 | ) | (29,434,119 | ) | 2,069,183 | ||||||||||||||||||||||
| Employee share-based compensation |
— | — | — | — | 117,900 | — | — | — | — | 117,900 | ||||||||||||||||||||||||
| Accrued interest on note receivable |
— | — | — | — | — | — | — | (21,659 | ) | — | (21,659 | ) | ||||||||||||||||||||||
| Conversion of convertible note payable |
— | — | 417,960 | 4,180 | 1,495,820 | — | — | — | — | 1,500,000 | ||||||||||||||||||||||||
| Net loss for the year |
— | — | — | — | — | — | — | — | (5,429,785 | ) | (5,429,785 | ) | ||||||||||||||||||||||
| Balances, December 31, 2008 |
7,965,000 | 7,965,000 | 5,451,777 | 54,518 | 25,653,645 | — | — | (573,620 | ) | (34,863,904 | ) | (1,764,361 | ) | |||||||||||||||||||||
| Employee share-based compensation |
— | — | — | — | 130,587 | — | — | — | — | 130,587 | ||||||||||||||||||||||||
| Accrued interest on notes receivable |
— | — | — | — | — | — | — | (57,779 | ) | — | (57,779 | ) | ||||||||||||||||||||||
| Purchase of treasury stock for cash |
— | — | (129,962 | ) | — | — | (547,835 | ) | — | — | — | (547,835 | ) | |||||||||||||||||||||
| Issuance of note receivable, stockholder |
— | — | — | — | — | — | — | (500,000 | ) | — | (500,000 | ) | ||||||||||||||||||||||
| Options exercised for cash |
— | — | 3,333 | 33 | 10,630 | — | — | — | — | 10,663 | ||||||||||||||||||||||||
| Purchases of treasury stock through cancellation of notes and accrued interest |
— | — | (195,868 | ) | — | — | (1,131,399 | ) | — | 1,131,399 | — | — | ||||||||||||||||||||||
| Net loss for the year |
— | — | — | — | — | — | — | — | (7,159,060 | ) | (7,159,060 | ) | ||||||||||||||||||||||
| Balances, December 31, 2009 |
7,965,000 | $ | 7,965,000 | 5,129,280 | $ | 54,551 | $ | 25,794,862 | $ | (1,679,234 | ) | $ | — | $ | — | $ | (42,022,964 | ) | $ | (9,887,785 | ) | |||||||||||||
| Employee share-based compensation |
— | — | — | — | 53,820 | — | — | — | — | 53,820 | ||||||||||||||||||||||||
| Fair value of conversion feature of notes payable |
— | — | — | — | 834,555 | — | — | — | — | 834,555 | ||||||||||||||||||||||||
| Warrants issued in connection with senior unsecured convertible notes |
— | — | — | — | 120,218 | — | — | — | — | 120,218 | ||||||||||||||||||||||||
| Net loss for the three months |
— | — | — | — | — | — | — | — | (2,515,712 | ) | (2,515,712 | ) | ||||||||||||||||||||||
| Balances, March 31, 2010 |
7,965,000 | $ | 7,965,000 | 5,129,280 | $ | 54,551 | $ | 26,803,455 | $ | (1,679,234 | ) | $ | — | $ | — | $ | (44,538,676 | ) | $ | (11,394,904 | ) | |||||||||||||
See notes to financial statements.
F-5
Statements of Cash Flows
| (unaudited) Three Months Ended March 31, |
Years
Ended December 31, |
|||||||||||||||||||
| 2010 | 2009 | 2009 | 2008 | 2007 | ||||||||||||||||
| Cash flows from operating activities: |
||||||||||||||||||||
| Net loss |
$ | (2,515,712 | ) | $ | (1,424,913 | ) | $ | (7,159,060 | ) | $ | (5,429,785 | ) | $ | (3,697,137 | ) | |||||
| Adjustments to reconcile net loss to net cash flows from operating activities: |
||||||||||||||||||||
| Depreciation and license amortization |
47,035 | 39,064 | 168,710 | 84,484 | 16,728 | |||||||||||||||
| Expenses paid through the issuance of common stock |
— | — | — | — | 57,784 | |||||||||||||||
| Share-based compensation |
53,820 | 38,089 | 130,587 | 117,900 | 20,401 | |||||||||||||||
| Change in fair value of derivative liability |
209,350 | — | — | — | — | |||||||||||||||
| Amortization of debt issuance costs and original issue discount |
93,173 | — | 98,500 | 394,738 | 394,737 | |||||||||||||||
| Accrued interest on notes receivable, stockholder |
— | (12,067 | ) | (57,779 | ) | (21,659 | ) | (21,600 | ) | |||||||||||
| Increase (decrease) in cash resulting from changes in: |
||||||||||||||||||||
| Due from related parties |
204 | — | 8,113 | (8,317 | ) | 1,864 | ||||||||||||||
| Inventory |
(77,081 | ) | (175,000 | ) | (569,350 | ) | — | — | ||||||||||||
| Prepaid expenses and other current assets |
(80,803 | ) | 19,634 | (33,383 | ) | (21,440 | ) | 4,682 | ||||||||||||
| Deposits |
— | 1,926 | 4,775 | (24,226 | ) | (38,033 | ) | |||||||||||||
| Accounts payable and accrued expenses |
287,934 | (256,203 | ) | 418,970 | 603,975 | 141,154 | ||||||||||||||
| Related party deferred revenue |
(650,000 | ) | (650,000 | ) | (2,488,725 | ) | 11,560,099 | 125,000 | ||||||||||||
| Net cash flows from operating activities |
(2,632,080 | ) | (2,419,470 | ) | (9,478,642 | ) | 7,255,769 | (2,994,420 | ) | |||||||||||
| Cash flows from investing activities: |
||||||||||||||||||||
| Purchases of furniture, software and equipment |
(33,153 | ) | (153,922 | ) | (282,362 | ) | (856,782 | ) | (62,179 | ) | ||||||||||
| Purchase of licenses |
— | — | — | (90,000 | ) | — | ||||||||||||||
| Net cash flows from investing activities |
(33,153 | ) | (153,922 | ) | (282,362 | ) | (946,782 | ) | (62,179 | ) | ||||||||||
| Cash flows from financing activities: |
||||||||||||||||||||
| Proceeds from senior unsecured convertible notes, net of issuance costs |
3,777,142 | — | — | — | — | |||||||||||||||
| Purchase of treasury stock for cash |
— | (500,000 | ) | (547,835 | ) | — | — | |||||||||||||
| Issuance of note receivable, stockholder |
— | (500,000 | ) | (500,000 | ) | — | — | |||||||||||||
| Deferred offering costs paid |
(132,319 | ) | — | (53,496 | ) | — | — | |||||||||||||
| Proceeds from related party convertible note |
— | — | 3,500,000 | — | 500,000 | |||||||||||||||
| Proceeds from option exercises |
— | — | 10,663 | — | — | |||||||||||||||
| Proceeds from Series A preferred stock offering |
— | — | — | — | 100,000 | |||||||||||||||
| Net cash flows from financing activities |
3,644,823 | (1,000,000 | ) | 2,409,332 | — | 600,000 | ||||||||||||||
| Net change in cash |
979,590 | (3,573,392 | ) | (7,351,672 | ) | 6,308,987 | (2,456,599 | ) | ||||||||||||
| Cash, beginning of period |
2,569,129 | 9,920,801 | 9,920,801 | 3,611,814 | 6,068,413 | |||||||||||||||
| Cash, end of period |
$ | 3,548,719 | $ | 6,347,409 | $ | 2,569,129 | $ | 9,920,801 | $ | 3,611,814 | ||||||||||
| SUPPLEMENTAL CASH FLOW INFORMATION |
||||||||||||||||||||
| Cash paid for: |
||||||||||||||||||||
| Income taxes |
$ | 49,250 | — | — | — | — | ||||||||||||||
| Interest |
— | — | — | — | — | |||||||||||||||
See notes to financial statements.
F-6
SURGIVISION, INC.
Statements of Cash Flows (Continued)
NON-CASH TRANSACTIONS:
| • | In 2007, warrants were issued with a fair value of $789,475 as part of the amendment to the convertible note payable |
| • | In 2008, convertible notes payable of $1,500,000 were converted into 417,960 shares of common stock |
| • | In December of 2009, related party notes receivable and accrued interest in the amount of $1,131,399 were cancelled in exchange for 195,868 shares of treasury stock |
| • | At March 31, 2010 and December 31, 2009, deferred offering costs in the amount of $550,575 and $313,007, respectively, were included in accrued expenses. |
| • | In March 2010, warrants (recorded as deferred financing costs and additional paid-in capital) were issued with a fair value of $120,218 to the placement agent in connection with the senior unsecured convertible notes |
See notes to financial statements.
F-7
Notes to Financial Statements
Years Ended December 31, 2009, 2008 and 2007 and the
Unaudited Three Month Periods Ended March 31, 2010 and 2009
Note 1 – Formation and Nature of Business
SurgiVision, Inc. (the “Company”), a Delaware corporation, was formed on March 12, 1998. The Company operates in the medical device industry and is focused on the development and commercialization of technology that enables physicians to see inside the brain and heart using direct, intra-procedural magnetic resonance imaging, or MRI, guidance while performing minimally invasive surgical procedures. Prior to 2008, the Company was a development stage entity.
The Company’s first product candidate is the ClearPoint system, which is designed to allow minimally invasive procedures in the brain to be performed in an MRI suite. The Company’s second product candidate is the ClearTrace system, which is designed to allow catheter-based minimally invasive procedures in the heart to be performed in an MRI suite. The Company is also pursuing what it refers to as its SafeLead Development Program, the purpose of which is to incorporate the Company’s MRI-safety technologies into a third party’s implantable leads for cardiac and neurological applications.
Note 2 – Significant Accounting Policies
Principles of Consolidation—The financial statements include SurgiVision, Inc. and its approximate 93% owned subsidiary, Cardiac EP Sub, Inc., a Delaware corporation, which was formed on December 19, 2008. The minority interest associated with the investment in Cardiac EP Sub, Inc. is of nominal value as of March 31, 2010 and December 31, 2009 and 2008, and consequently, has not been recognized in the financial statements. All significant intercompany balances and transactions have been eliminated in the financial statements.
Unaudited Interim Financial Information—The accompanying balance sheet as of March 31, 2010, the statements of operations and cash flows for the three months ended March 31, 2010 and 2009, and the statement of stockholders’ equity for the three months ended March 31, 2010 are unaudited. These unaudited interim financial statements have been prepared in accordance with accounting principles generally accepted in the United States. In the opinion of the Company’s management, these unaudited interim financial statements have been prepared on the same basis as the audited financial statements and include all adjustments necessary for the fair presentation of the Company’s financial position, and the related statements of operations, stockholders’ equity and cash flows for the interim periods presented. The results for the three months ended March 31, 2010 are not necessarily indicative of the results to be expected for the year ended December 31, 2010.
Use of Estimates—The preparation of financial statements in conformity with accounting principles generally accepted in the United States requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Actual results could differ from those estimates.
Concentration of Credit Risk—The Company places its cash on deposit with financial institutions in the United States. In October and November 2008 the Federal Deposit Insurance Corporation (“FDIC”) temporarily increased coverage to $250,000 for substantially all depository accounts and temporarily provides unlimited coverage for certain qualifying and participating non-interest bearing transaction accounts. The increased coverage is scheduled to expire on December 31, 2013, at which time it is anticipated amounts insured by the FDIC will return to $100,000. From time to time, the Company may have amounts on deposit in excess of the insured limits. As of March 31, 2010, the Company had approximately $98,000 of cash and cash equivalents which exceeded these insured amounts.
F-8
SURGIVISION, INC.
Notes to Financial Statements
Years Ended December 31, 2009, 2008 and 2007 and the
Unaudited Three Month Periods Ended March 31, 2010 and 2009
Note 2 – Significant Accounting Policies – (continued)
Cash and Cash Equivalents—Cash and cash equivalents include all highly liquid investments with an original maturity of three months or less.
Inventory—Inventory is carried at the lower of cost (first-in, first-out (“FIFO”) method) or net realizable value. All items included in inventory relate to the Company’s ClearPoint system. As of March 31, 2010, the ClearPoint system has not received regulatory clearance from the Food and Drug Administration (the “FDA”) for commercial sale in the United States. If the Company is unable to obtain clearance, these amounts will be charged to expense to the extent that the inventory cannot be returned to the vendors for cash or sold for scrap. At each reporting period in which the balance sheet reflects inventory related to products that do not have regulatory clearance or approval, the Company evaluates the likelihood of receiving regulatory clearance or approval for these products based on input from the Company’s external regulatory advisers. The Company also considers its anticipated selling prices based on analysis of product pricing of competitors and review of market information prepared by third party research analysts to determine net realizable value.
Inventory consists of the following amounts related to the Company’s ClearPoint system as of March 31, 2010 and December 31, 2009:
| March 31, 2010 |
December 31, 2009 | |||||
| Software licenses (Note 9) |
$ | 175,000 | $ | 175,000 | ||
| Hardware |
321,047 | 268,447 | ||||
| Disposable components—work in process |
150,384 | 125,903 | ||||
| $ | 646,431 | $ | 569,350 | |||
Furniture, Software and Equipment—Furniture, software and equipment are recorded at cost and are depreciated on a straight-line basis over their estimated useful lives, principally five to seven years. Leasehold improvements are depreciated on a straight line basis over the lesser of the estimated useful lives or the life of the related lease.
Licenses—Licenses are recorded at cost and are amortized using the straight-line method over their estimated useful life. The carrying value of licenses at March 31, 2010, and December 31, 2009 and 2008 was $58,500, $63,000 and $81,000, respectively, net of accumulated amortization of $31,500, $27,000 and $9,000, for the same periods, respectively. Future amortization under licenses is expected to be approximately $18,000 annually through June 2013. One of the licenses contains a requirement to pay the licensor an additional $40,000 upon the issuance of a certain patent, and a second license contains a requirement to pay the licensor an additional $20,000 upon the issuance of another patent. The license arrangements require certain minimum royalty payments to the licensor. As of December 31, 2010, future minimum royalty payments are as follows:
| Years ending December 31, | |||
| 2010 |
$ | 45,000 | |
| 2011 |
70,000 | ||
| 2012 |
70,000 | ||
| 2013 |
95,000 | ||
| 2014 |
95,000 | ||
| Thereafter |
1,200,000 | ||
| $ | 1,575,000 | ||
F-9
SURGIVISION, INC.
Notes to Financial Statements
Years Ended December 31, 2009, 2008 and 2007 and the
Unaudited Three Month Periods Ended March 31, 2010 and 2009
Note 2 – Significant Accounting Policies – (continued)
Royalty payment amounts may be greater than the above amounts based on the negotiated royalty rates. If the Company sublicenses the intellectual property that is licensed from the licensor and the Company receives any payment under or with respect to such sublicense, the Company is obligated to pay the licensor an agreed upon percentage of any such payment(s). Under the terms of these license agreements, the Company is required to reimburse the licensor for all costs associated with patent filing, prosecution and maintenance as well as expenses related to enforcing the related patent rights.
The Company may terminate these license agreements for any reason, upon giving the licensor either 60 or 90 days’ written notice, depending on the agreement. One of the licenses is cancelable by the licensor if, by the fourth anniversary of the effective date (June 30, 2012), there have been no commercial sales of a product subject to the license.
Impairment of Long-Lived Assets—The Company evaluates the recoverability of its long-lived assets (finite-lived intangible assets and furniture, software and equipment) whenever events or changes in circumstances indicate that the carrying amount of long-lived assets may not be fully recoverable. When this occurs, the expected undiscounted future cash flows are compared to the net book value of the related assets. If the net book value of the related assets exceeds the undiscounted expected future cash flows of the assets, the carrying amount would be reduced to the present value of the expected future cash flows and an impairment loss would be recognized. There have been no impairment losses in the periods presented.
Revenue Recognition—The Company analyzes revenue recognition on an agreement by agreement basis as discussed herein.
| • | Related Party Revenue Recognition under Boston Scientific Corporation “BSC” Neuro Agreement (Note 5)—The Company analyzed whether the components of the arrangement represent separate units of accounting as defined by Accounting Principles Generally Accepted in the United States (“GAAP”). Application of these standards requires subjective determinations and requires management to make judgments about the value of the individual elements and whether delivered elements are separable from the other aspects of the contractual relationship. The Company determined it did not have clear and objective evidence of fair value of the various elements of the agreement and therefore, under GAAP regarding Multiple-Element Arrangements, has determined that the deliverables will be accounted for as one unit of accounting. |
This agreement requires specified milestones in the development of an MRI-safe implantable lead to be achieved by December 31, 2012. If the milestones are not achieved by that date and this failure is not the result of BSC Neuro’s failure to reasonably cooperate with the Company in pursuing the milestones, the Company will be required to repay BSC Neuro certain amounts, including any development expenses and milestone payments previously made to the Company under this agreement and any patent prosecution costs incurred by BSC Neuro with respect to the intellectual property licensed under this agreement. The existence of this provision indicates the sales price is not fixed or determinable and all monies which have been or will be received prior to December 31, 2012 have and will be deferred until such time. If the repayment obligations are not triggered as of December 31, 2012, the related party deferred revenue related to this contract will be recognized over the estimated period of continuing involvement. If the repayment obligations are triggered as of December 31, 2012, the related party deferred revenue related to this contract will be repaid to BSC Neuro.
F-10
SURGIVISION, INC.
Notes to Financial Statements
Years Ended December 31, 2009, 2008 and 2007 and the
Unaudited Three Month Periods Ended March 31, 2010 and 2009
Note 2 – Significant Accounting Policies – (continued)
The agreement includes research and development services requirements. The Company has recognized deferred research and development services revenue along with the related costs (charged to expense) on a gross basis since the Company is obligated and bears all credit risk with respect to the cost of providing the services.
Future product royalty payments related to the agreement will be recognized as the related products are sold and payments are due to the Company.
| • | Related Party Revenue Recognition under BSC Cardiac Agreement (Note 5)—The Company analyzed whether the components of the arrangement represent separate units of accounting as defined by GAAP. Application of these standards requires management to make subjective judgments about the value of the individual elements and whether delivered elements are separable from the other aspects of the contractual relationship. |
The Company defers recognition of non-refundable upfront license fees if there are continuing performance obligations without which the technology, know-how, rights, products or services conveyed in conjunction with the non-refundable fees have no utility to the licensee that could be considered separate and independent of the Company’s performance under other elements of the arrangement. Since the Company has continuing involvement through research and development services that is required because the Company’s know-how and expertise related to the technology are proprietary to the Company, or can only be performed by the Company, such upfront fees are deferred and recognized over the estimated period of continuing involvement on a straight line basis.
Payments to be received related to substantive, performance-based milestones in research and development arrangements are deferred upon receipt and achievement of the milestones as specified in the underlying agreement and recognized over the period of continuing involvement.
Future product royalty payments related to the agreement will be recognized as the related products are sold and payments are due to the Company.
Research and Development Costs—Costs related to research, design and development of products are charged to research and development expense as incurred. These costs include direct salary costs for research and development personnel, costs for materials used in research and development activities and costs for outside services.
Income Taxes—Deferred tax assets and liabilities are recognized for the estimated future tax consequences attributable to differences between the financial statement carrying amounts of existing assets and liabilities and their respective income tax bases. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. The effect on deferred tax assets and liabilities of a change in tax rates is recognized in the period that included the enactment date.
Valuation allowances are recorded for deferred tax assets when the recoverability of such assets is not deemed more likely than not.
Management has evaluated the effect of the guidance provided by GAAP regarding accounting for uncertainty in income taxes that became effective in 2009. In that regard, management has evaluated all tax positions that could have a significant effect on the financial statements and determined the Company has no
F-11
SURGIVISION, INC.
Notes to Financial Statements
Years Ended December 31, 2009, 2008 and 2007 and the
Unaudited Three Month Periods Ended March 31, 2010 and 2009
Note 2 – Significant Accounting Policies – (continued)
uncertain tax positions at March 31, 2010 or December 31, 2009. The Company’s tax returns after 2005 remain open for examination.
Net Loss Per Share—Basic net loss per common share is computed by dividing the net loss for the period by the weighted average number of common shares outstanding during the period. For all periods presented, diluted net loss per share is the same as basic net loss per share, as the inclusion of equivalent shares from outstanding common stock options, warrants, convertible debt and preferred stock would be anti-dilutive. The following table sets forth potential shares of common stock that are not included in the calculation of diluted net loss per share because to do so would be anti-dilutive as of the end of each period presented:
| Three months ended March 31, | Years ended December 31, | |||||||||
| 2010 | 2009 | 2009 | 2008 | 2007 | ||||||
| Stock options |
667,277 | 602,375 | 669,777 | 599,875 | 451,250 | |||||
| Warrants |
435,986 | 828,501 | 410,542 | 828,501 | 828,501 | |||||
| Convertible preferred shares |
1,991,250 | 1,991,250 | 1,991,250 | 1,991,250 | 1,991,250 | |||||
| Shares under convertible note agreements |
964,083 | — | 444,247 | — | 417,960 | |||||
| 4,058,596 | 3,422,126 | 3,515,816 | 3,419,626 | 3,688,961 | ||||||
Share-Based Compensation—The Company accounts for compensation for all arrangements under which employees and others receive shares of stock or equity instruments (including options and warrants) in accordance with FASB ASC Topic 718 “Compensation – Stock Compensation”, or ASC Topic 718. Under ASC Topic 718, the fair value of each award is estimated and amortized as compensation expense over the requisite service period. The fair value of the Company’s share-based options and warrants is estimated on the grant date using the Black-Scholes valuation model. This valuation model requires the input of highly subjective assumptions, including the expected price volatility and estimated option term. As the Company has been operating as a private company, it was unable to use actual price volatility and option life data as input assumptions within its Black-Scholes valuation model. Prior to October 2009, the Company used expected volatilities based on the historical volatility of the industry sector in which the Company operates, in accordance with the guidance set forth in ASC Topic 718.
Beginning in October 2009, the Company based its estimate of expected volatility on the average of historical volatilities of publicly traded companies it deemed similar because the Company lacks its own relevant historical volatility data. The Company will consistently apply this methodology until a sufficient amount of historical information regarding the volatility of the Company’s own share price becomes available.
To estimate the expected term, the Company utilizes the “simplified” method for “plain vanilla” options as discussed within the Securities and Exchange Commission’s Staff Accounting Bulletin 107, or SAB 107. The Company believes that all factors listed within SAB 107 as pre-requisites for utilizing the simplified method are true for the Company and for the Company’s share-based compensation arrangements. The Company intends to utilize the simplified method for the foreseeable future until more detailed information about exercise behavior becomes available.
The Company’s risk-free interest rates are based on a zero-coupon U.S. treasury instrument, the term of which is consistent with the expected term of the stock options. The Company has not paid and does not anticipate paying cash dividends on its shares of common stock; therefore, the expected dividend yield is assumed to be zero.
F-12
SURGIVISION, INC.
Notes to Financial Statements
Years Ended December 31, 2009, 2008 and 2007 and the
Unaudited Three Month Periods Ended March 31, 2010 and 2009
Note 2 – Significant Accounting Policies – (continued)
Fair Value Determination of Privately-Held Equity Securities—The fair values of the common stock as well as the common stock underlying options and warrants granted as compensation, or issued in connection with the settlement of liabilities, were estimated by management, with input from a third-party valuation specialist.
Determining the fair value of stock requires making complex and subjective judgments. The Company has used the income approach, the market approach, and the probability weighted expected return method to estimate the value of the enterprise for the dates on which securities are issued/granted and outstanding. The income approach was based on estimated future cash flows that utilized the Company’s forecasts of revenue and costs. The assumptions underlying the revenue and cost estimates are consistent with the Company’s business plan. The market approach was based on recent sales of the Company’s common stock in privately negotiated transactions between stockholders. Once the Company began the process of preparing for its initial public offering of common stock, the Company began to utilize the probability weighted expected return method, which is based on identifying the most likely liquidity events for the Company, the probability of each occurring, and the equity values for each after applying different percentages to the likelihood of the different values assigned to each anticipated outcome of those events. The assumptions used in each of the different valuation methods take into account certain discounts such as selecting the appropriate discount rate and control and lack of marketability discounts. The discount rates used in these valuations ranged from 22% to 35%. The discounts for lack of marketability ranged from 15% to 35% and the discount for lack of control ranged from 20% to 30%. If different discount rates or lack of marketability and control discounts had been used, the valuations would have been different. The enterprise value under each valuation method was allocated to preferred and common shares taking into account the enterprise value available to all stockholders and allocating that value among the various classes of stock based on the rights, privileges, and preferences of the respective classes in order to provide an estimate of the fair value of a share of the Company’s common stock. There is inherent uncertainty in these estimates.
Fair Value Measurements—Effective January 1, 2008, the Company adopted the provisions of a required new accounting standard related to fair value accounting for financial assets and liabilities, as well as for any other assets and liabilities that are carried at fair value on a recurring basis. As required, the Company adopted this standard for all non-financial assets and liabilities that are recognized or disclosed at fair value on a recurring basis, as of January 1, 2009. The adoption did not materially impact the Company’s consolidated financial position and results of operations.
Fair value in this standard is defined as the exchange price that would be received for an asset or paid to transfer a liability (an exit price) in the principal or most advantageous market for the asset or liability in an orderly transaction between market participants on the measurement date. The standard also establishes a fair value hierarchy, which requires an entity to maximize the use of observable inputs and minimize the use of unobservable inputs when measuring fair value. Three levels of inputs may be used to measure fair value:
Level 1 – quoted prices in active markets for identical assets or liabilities
Level 2 – quoted prices for similar assets and liabilities in active markets or inputs that are observable
Level 3 – inputs that are unobservable (for example cash flow modeling inputs based on assumptions)
F-13
SURGIVISION, INC.
Notes to Financial Statements
Years Ended December 31, 2009, 2008 and 2007 and the
Unaudited Three Month Periods Ended March 31, 2010 and 2009
Note 2 – Significant Accounting Policies – (continued)
The following table summarizes liabilities measured at fair value on a recurring basis:
| Fair Value Measurements Using | ||||||||||||
| Level 1 | Level 2 | Level 3 | Total | |||||||||
| Liabilities |
||||||||||||
| Derivative liability, Note 6 (December 31, 2009) |
$ | — | $ | — | $ | 1,227,500 | $ | 1,227,500 | ||||
| Derivative liability, Note 6 (March 31, 2010) |
$ | — | $ | — | $ | 1,436,850 | $ | 1,436,850 | ||||
The following table summarizes changes in Level 3 Liabilities measured at fair value on a recurring basis:
| Level 3 Liabilities | |||
| Balance as of December 31, 2008 |
$ | — | |
| Issuance of derivative liability, Note 6 |
1,227,500 | ||
| Balance as of December 31, 2009 |
1,227,500 | ||
| Loss on change in fair value |
209,350 | ||
| Balance as of March 31, 2010 |
$ | 1,436,850 | |
The financial instruments recorded in the balance sheets include cash and cash equivalents, accounts payable, related party convertible notes, and senior unsecured convertible notes. Due to their short-term maturity, the carrying amounts of cash and cash equivalents and accounts payable approximate their fair value. At March 31, 2010, the fair value of the related party convertible notes payable is $3,500,000 and the fair value of senior unsecured convertible notes is $4,071,000.
Derivative Financial Instruments. The Company accounts for derivative instruments in accordance with FASB ASC Topic 815, which establishes accounting and reporting standards for derivative instruments and hedging activities, including certain derivative instruments embedded in other financial instruments or contracts and requires recognition of all derivatives on the balance sheet at fair value. Changes in the fair value of derivatives are recorded each period as a gain or loss in the statement of operations unless the derivative qualifies for hedge accounting. At March 31, 2010, and December 31, 2009 and 2008, the Company did not have any derivative instruments that were designated as hedges. (See Note 6)
New Accounting Pronouncements. In August 2009, the FASB issued ASU No. 2009-04, Accounting for Redeemable Equity Instruments—Amendment to Section 480-10-S99, or ASU No. 2009-04. This ASU represents an update to Section 480-10-S99, Distinguishing Liabilities from Equity , per Emerging Issues Task Force Topic D-98, “Classification and Measurement of Redeemable Securities.” The adoption of ASU 2009-04 did not have a material impact on the Company’s financial statements.
In August 2009, the FASB issued ASU No. 2009-05, Fair Value Measurements and Disclosures (Topic 820)—Measuring Liabilities at Fair Value, or ASU No. 2009-05. This ASU amends Subtopic 820-10, Fair Value Measurements and Disclosures—Overall, to provide guidance on the fair value measurement of liabilities. The adoption of ASU 2009-05 did not have a material impact on the Company’s financial statements.
In October 2009, the Financial Accounting Standards Board (“FASB”) issued Accounting Standards Update No. 2009-13 (“ASU 2009-13”), which addresses the accounting for multiple-deliverable arrangements to enable
F-14
SURGIVISION, INC.
Notes to Financial Statements
Years Ended December 31, 2009, 2008 and 2007 and the
Unaudited Three Month Periods Ended March 31, 2010 and 2009
Note 2 – Significant Accounting Policies – (continued)
vendors to account for products or services (deliverables) separately rather than as a combined unit. ASU 2009-13 is effective prospectively for revenue arrangements entered into or materially modified beginning in fiscal years on or after June 15, 2010. Early adoption is permitted. The Company is currently evaluating the impact that the adoption of this standard will have on its financial statements, if any.
In February 2010, the FASB issued authoritative guidance that amends the disclosure requirements related to subsequent events. This guidance includes the definition of a Securities and Exchange Commission filer, removes the definition of a public entity, redefines the reissuance disclosure requirements and allows companies to omit the disclosure of the date through which subsequent events have been evaluated. This guidance is effective for financial statements issued for interim and annual periods ending after February 2010. This guidance did not materially impact the Company’s results of operations or financial position, but did require changes to the Company’s disclosures in its financial statements.
In April 2010, the FASB issued Accounting Standards Update No. 2010-17 (“ASU 2010-17”) which provides guidance on defining a milestone and determining when it may be appropriate to apply the milestone method of revenue recognition for research or development arrangements that contain payment provisions contingent upon achieving specified events. ASU 2010-17 is effective for milestones achieved in fiscal years beginning on or after June 15, 2010. Early adoption is permitted. The Company is currently evaluating the impact that the adoption of this standard will have on its financial statements, if any.
Note 3 – Liquidity and Management’s Plans
The accompanying financial statements have been prepared assuming the Company will continue as a going concern. For the three months ended March 31, 2010 and the years ended December 31, 2009, 2008 and 2007, the Company incurred net losses of $2,515,712, $7,159,060, $5,429,785, and $3,697,137, respectively, and the cumulative net loss since the Company’s inception through March 31, 2010 is $44,538,676. In view of these matters, the ability of the Company to continue as a going concern is dependent upon the Company’s ability to generate additional financing sufficient to support its research and development activities, clearance or approval of developed products for sale by applicable regulatory authorities, including the FDA, and ultimately to generate revenue sufficient to cover all costs. Since inception, the Company has financed its activities principally from the sale of equity securities, borrowings, and license arrangements. The Company intends on financing its future development activities and its working capital needs largely from the sale of equity securities until such time that funds provided by operations are sufficient to fund working capital requirements. There can be no assurance that the Company will be successful at achieving its financing goals on reasonable commercial terms, if at all, or if it will generate revenues sufficient to cover its costs.
F-15
SURGIVISION, INC.
Notes to Financial Statements
Years Ended December 31, 2009, 2008 and 2007 and the
Unaudited Three Month Periods Ended March 31, 2010 and 2009
Note 4 – Furniture, Software and Equipment
Furniture, software and equipment consist of the following:
| March
31, 2010 |
December 31, | |||||||||||
| 2009 | 2008 | |||||||||||
| Furniture and equipment |
$ | 1,112,193 | $ | 1,079,030 | $ | 807,012 | ||||||
| Software |
19,222 | 19,232 | 8,888 | |||||||||
| Leasehold Improvements |
157,236 | 157,236 | 157,236 | |||||||||
| 1,288,651 | 1,255,498 | 973,136 | ||||||||||
| Less accumulated depreciation |
(305,875 | ) | (263,340 | ) | (112,630 | ) | ||||||
| $ | 982,776 | $ | 992,158 | $ | 860,506 | |||||||
Depreciation expense was as follows:
| Three Months Ended March 31, | Years Ended December 31, | |||||||||||||
| 2010 | 2009 | 2009 | 2008 | 2007 | ||||||||||
| $42,535 | $ | 34,564 | $ | 150,710 | $ | 71,928 | $ | 16,728 | ||||||
Note 5 – Related Party License Agreements
License and development agreements have been entered into with affiliates of Boston Scientific Corporation (“BSC”). Because BSC’s affiliate is a stockholder and has a representative on the Company’s board of directors, management has deemed all transactions with BSC and its affiliates to be of a related party nature.
BSC Neuro Agreement—On December 30, 2005, the Company entered into definitive license and development agreements (collectively, as amended, the “BSC Neuro Agreement”) with Advanced Bionics Corporation, an affiliate of BSC. Advanced Bionics Corporation subsequently changed its name to Boston Scientific Neuromodulation Corporation (“BSC Neuro”). Under the BSC Neuro Agreement, the Company granted BSC Neuro an exclusive commercial license with respect to certain of the Company’s owned and licensed intellectual property, in the neuromodulation field, to make, use, import, lease and sell neuro-related leads, neuro-related lead extensions, and neuro-related lead-type devices, such as implantable pulse generators. The Company has continuing research and development obligations pursuant to the BSC Neuro Agreement with respect to the development of MRI-compatible and MRI-safe implantable neuromodulation leads.
Under the BSC Neuro Agreement, in addition to prospective royalty payments on net sales of licensed products, the Company could receive up to $1,600,000 in future milestone-based payments associated with successful development and regulatory approval of the leads. The Company did not receive any up-front license payments pursuant to this agreement. In addition, the Company could receive over $500,000 in incentive payments for incremental development work BSC Neuro may request. This agreement requires specified milestones in the development of an MRI-safe implantable lead to be achieved by December 31, 2012. If the milestones are not achieved by that date and this failure is not the result of BSC Neuro’s failure to reasonably cooperate with the Company in pursuing the milestones, the Company will be required to repay BSC Neuro certain amounts, including any development expenses and milestone payments previously made to the Company under this agreement and any patent prosecution costs incurred by BSC Neuro with respect to the intellectual
F-16
SURGIVISION, INC.
Notes to Financial Statements
Years Ended December 31, 2009, 2008 and 2007 and the
Unaudited Three Month Periods Ended March 31, 2010 and 2009
Note 5 – Related Party License Agreements – (continued)
property licensed under this agreement. As of March 31, 2010, the Company had received approximately $750,000 of payments from BSC Neuro which would be subject to the repayment obligation described above. In addition, the Company would be responsible to reimburse BSC Neuro for out of pocket costs incurred by BSC Neuro in prosecuting patent applications and maintaining issued patents for the licensed technologies. As discussed in Note 2, Revenue Recognition, all amounts received have been recorded as deferred revenues.
BSC Cardiac Pacemakers Agreement—Effective March 19, 2008, the Company entered into definitive license and development agreements (collectively the “BSC Cardiac Agreement”) with Cardiac Pacemakers, Inc. (“BSC Cardiac”), an affiliate of Boston Scientific Corporation. Under the BSC Cardiac Agreement, the Company granted BSC Cardiac an exclusive commercial license with respect to certain of the Company’s owned and licensed intellectual property rights, in the field of implantable medical leads for cardiac applications, to make, have made, use, promote, market, import, distribute, lease, sell, offer for sale and commercialize products in the licensed field of use. The Company is required to continue to investigate the feasibility of its technology and, upon successful completion of feasibility studies, to work with BSC Cardiac to develop this technology for different types of MRI-compatible and MRI-safe implantable cardiac leads.
Pursuant to the BSC Cardiac Agreement, in addition to prospective royalty payments on net sales of licensed products, the Company received non-refundable licensing fees totaling $13,000,000 in 2008, and the Company could receive up to $20,000,000 in future milestone-based payments associated with the successful development and regulatory approval of the implantable cardiac leads, subject to certain patents being issued on patent applications licensed to BSC Cardiac. The Company initially recorded the payment as deferred revenue and is subsequently recognizing revenue over the five year estimated period of continuing involvement (see Note 2, Revenue Recognition). The Company determined the five year estimated period of continuing involvement based upon the Company’s internal development plan and projected timeline for the different implantable cardiac leads.
Except as set forth below, the licensing provisions of the BSC Cardiac Agreement will terminate upon the expiration of the last issued patent that is licensed under the agreement, and the development provisions of the BSC Cardiac Agreement will expire upon FDA approval of a design for each of the different lead types described in the agreement. BSC Cardiac has the one-time option, within 60 days after successful completion of the first cardiac lead feasibility study, to cease further development work and to terminate the provisions of the BSC Cardiac Agreement. If BSC Cardiac elects to exercise its option under the BSC Cardiac Agreement to terminate further development efforts, the license the Company granted to BSC Cardiac will automatically become non-exclusive with respect to some intellectual property, other intellectual property will be removed from the scope of the license and revert to the Company, and BSC Cardiac will not be obligated to pay the Company any future royalties on net sales of products containing intellectual property that remains subject to the non-exclusive license. Likewise, any unachieved future milestone-based payments will not be paid or due the Company. However, upon any such termination, the Company will not be required to repay any portion of the upfront licensing fees paid and would recognize any unamortized deferred revenue.
F-17
SURGIVISION, INC.
Notes to Financial Statements
Years Ended December 31, 2009, 2008 and 2007 and the
Unaudited Three Month Periods Ended March 31, 2010 and 2009
Note 5 – Related Party License Agreements – (continued)
Remaining related party deferred revenue is expected to be recognized as revenue as follows:
| Years ending December 31, (except as noted below) | |||
| 2010 (April through December) |
$ | 1,950,000 | |
| 2011 |
2,600,000 | ||
| 2012 |
2,600,000 | ||
| 2013 |
1,396,374 | ||
| $ | 8,546,374 | ||
Note 6 – Related Party Notes Payable
Related Party Convertible Notes Payable (BSC)—On October 16, 2009, the Company entered into a convertible note payable arrangement with BSC. The arrangement allowed for initial borrowings by the Company of $2,000,000, which was received in October 2009, and additional borrowings at future dates totaling up to $2,250,000. During November and December of 2009, the Company borrowed an additional $1,500,000 from BSC. All borrowings bear interest at 10% per annum and mature on the second anniversary of the date on which the funds were advanced (October through December 2011).
In addition, the Company will be required to prepay all or a portion of the loans upon the consummation of any qualified financing, which is any equity financing in which shares of the Company’s preferred stock are issued in exchange for cash proceeds. Upon consummation of a qualified financing from Medtronic, Inc., St. Jude Medical, Inc., or Johnson & Johnson, or any of their respective subsidiaries or affiliates, up to 100% of the cash proceeds from such qualified financing must be used to prepay the outstanding principal and accrued interest of the loans. Upon consummation of a qualified financing from any other investor, up to 25% of the cash proceeds from such qualified financing shall be applied by the Company to prepay the outstanding principal and accrued interest of the loans. The Company can prepay the loans at anytime. The principal and interest outstanding on each note is convertible, at the option of the holder, at any time prior to the earlier of the maturity date or the consummation of a qualified public offering (a bona fide first underwritten public offering of the Company’s common stock on a firm commitment basis in which the aggregate gross proceeds received by the Company at the public offering price equals or exceeds $20,000,000) into one share of the Company’s preferred stock at a conversion price equal to the lower of $8.00 per share, or the price per share paid by investors in a future preferred stock financing conducted by the Company prior to the qualified public offering. The notes are secured by a first priority security interest in all of the Company’s assets.
F-18
SURGIVISION, INC.
Notes to Financial Statements
Years Ended December 31, 2009, 2008 and 2007 and the
Unaudited Three Month Periods Ended March 31, 2010 and 2009
Note 6 – Related Party Notes Payable – (continued)
The Company analyzed the terms of the conversion feature of the notes under ASC Topic 815 and determined, based upon the conversion price reset provision, that the conversion feature should be accounted for as a derivative liability (Note 2, Fair Value Measurements). Under this guidance the conversion feature was initially measured at fair value and will be adjusted to the current fair value at each reporting period, changes in fair value will be recorded as other income (expense) in the related statement of operations. The Company calculated the fair value of this derivative liability utilizing the Black-Scholes pricing model. The assumptions used in calculating the fair value of the derivative liability using this model as of the transaction date and March 31, 2010 were as follows:
| March 31, 2010 | Transaction Date | |||||
| Dividend yield |
0 | % | 0 | % | ||
| Expected volatility |
52.77 | % | 38.28 | % | ||
| Risk free interest rate |
1.02 | % | 1.14 | % | ||
| Expected term |
1.55 years | 2 years |
There was no adjustment of the derivative liability at December 31, 2009 because the change in its fair value from the transaction date was insignificant. At March 31, 2010, the fair value of the derivative liability was $1,436,850; the change in fair value from December 31, 2009 in the amount of $209,350 was recorded as other expense in the statement of operations.
The proceeds from the transaction were allocated as follows:
| Financial Instrument |
|||
| Related party convertible notes payable |
$ | 2,272,500 | |
| Derivative liability |
1,227,500 | ||
| $ | 3,500,000 | ||
The discount on the related party convertible debt is amortized through charges to interest expense based upon the effective interest method through the date of maturity.
Related Party Convertible Notes Payable (BSC Neuro)—During December 2005, BSC Neuro advanced the Company $1,500,000 in the form of a convertible promissory note. The original maturity date of this note was December 31, 2007 or, if earlier, the expiration of a stipulated period of negotiations between BSC Neuro and the Company that followed the completion of certain product development work by the Company (the “Negotiation Period”).
The calculation of BSC Neuro’s conversion option under the note depended on whether BSC Neuro and the Company entered into a license agreement with respect to certain Company technology (the “Subsequent License”). If BSC Neuro and the Company did not enter into the Subsequent License, then the note was convertible into 10% of the Company’s fully diluted common shares (all outstanding common stock, all outstanding preferred stock convertible into shares of common stock, all warrants and options to acquire shares of common stock (vested and unvested) and all shares of common stock issuable under the Company’s equity compensation plans). If BSC Neuro and the Company did enter into the Subsequent License, then the note was convertible into 5% of the Company’s fully diluted common shares. There was no beneficial conversion feature associated with this transaction.
F-19
SURGIVISION, INC.
Notes to Financial Statements
Years Ended December 31, 2009, 2008 and 2007 and the
Unaudited Three Month Periods Ended March 31, 2010 and 2009
Note 6 – Related Party Notes Payable – (continued)
The note was amended on June 30, 2007, wherein the maturity date was extended to June 30, 2008 or, if earlier, the expiration of the Negotiation Period. The lender’s conversion option was then fixed at 5% of the Company’s fully diluted common shares. However, if at the time of conversion BSC Neuro and the Company had not entered into the Subsequent License, the Company was also required to issue BSC Neuro a warrant to purchase an additional 5% of the Company’s fully diluted common shares at an exercise price of $0.01 per share. Such warrant would only be exercisable if BSC Neuro and the Company did not enter into the Subsequent License by the end of the Negotiation Period. The conversion option under the amended note was substantively the same as the conversion option under the original note.
The June 30, 2007 amendment was evaluated to determine if it qualified as a debt extinguishment accounting. Based on the analysis performed, there was no debt extinguishment recorded as the fair value of the pre-amendment and post-amendment cash flows related to the notes did not differ by more than 10%. The fair value of the aforementioned $0.01 warrant of approximately $790,000 was recorded as a debt discount on the date of amendment and amortized through interest expense through the extended maturity date (June 30, 2008).
On June 30, 2008, BSC Neuro exercised its conversion option and converted the note in full into 417,960 shares of common stock. Upon conversion, BSC Neuro and the Company did not enter into the Subsequent License. Therefore, the number of shares subject to the aforementioned warrant was fixed at 417,960. The Negotiation Period expired during 2009, and BSC Neuro and the Company did not enter into the Subsequent License. BSC Neuro did not exercise the warrant and the warrant expired during 2009.
Note 7 – Senior Unsecured Convertible Notes
In March 2010, the Company issued 10% senior unsecured convertible notes, or the bridge notes, in the aggregate principal amount of $4,071,000. The bridge notes contain a mandatory conversion feature upon the closing of the initial public offering of the Company’s common stock that will automatically convert the bridge notes into shares of the Company’s common stock at the lesser of $8.00 per share or 80% of the offering price, subject to a $4.00 per share floor conversion price. In addition, holders of the bridge notes may convert the outstanding principal amount of their bridge notes into shares of the Company’s common stock at any time, based on a conversion price of $8.00 per share, subject to certain adjustments. The bridge notes mature two years from the date of issuance, unless earlier converted, and accrue interest at the rate of 10% per annum. All accrued interest will be paid in cash upon the earlier to occur of maturity or conversion and will not be converted into shares of the Company’s common stock.
The Company applied the guidance in ASC 815-40, “Derivatives and Hedging Contracts in an Entity’s Own Equity,” in determining that the conversion features of the bridge notes did not require derivative liability accounting treatment. The Company relied upon guidance in ASC 470-20, “Debt with Conversion and Other Options,” in determining that the non-mandatory conversion feature represented a beneficial conversion feature (the “BCF”) that should be recorded as equity based on its intrinsic value. The offset was recorded as a discount which was netted against the bridge notes. At the issuance dates of the bridge notes, the intrinsic value of the BCF was $834,555 which represents the difference between the fair value of $9.64 per common share and the conversion price of $8.00 per share multiplied by the number of conversion shares. The discount is being amortized to interest expense using the effective interest method over the term of the bridge notes.
F-20
SURGIVISION, INC.
Notes to Financial Statements
Years Ended December 31, 2009, 2008 and 2007 and the
Unaudited Three Month Periods Ended March 31, 2010 and 2009
Note 7 – Senior Unsecured Convertible Notes – (continued)
The Company incurred approximately $293,000 of costs related to the issuance of the bridge notes, comprised of placement agent commissions and legal fees. In addition, warrants with a five year term were issued to the placement agent exercisable for 25,444 shares of the Company’s common stock at a price equal to the lesser of $8.00 per share or 80% of the offering price in the Company’s initial public offering, subject to a $4.00 per share floor conversion price. The fair value of the placement agent warrants was approximately $120,000 (Note 8). The total costs incurred in connection with the issuance of the bridge notes of approximately $413,000 were capitalized as deferred financing costs and are being amortized using the effective interest method over the term of the bridge notes.
Note 8 – Stockholders’ Equity
Series A Preferred Stock—In 2006, the Company issued 7,965,000 shares of Series A Convertible Preferred Stock for net proceeds of $7,335,787 ($7,965,000 net of $629,213 in transaction expenses). Additionally, the placement agent received detachable warrants to acquire up to 141,500 shares of the Company’s common stock at $4.00 per share with a fair value of $28,696 on the date of issuance. The warrants expire on December 31, 2011.
The holders of the Series A Convertible Preferred Stock have the following rights and privileges.
Voting. Each holder of Series A Convertible Preferred Stock is entitled to vote on all matters presented to holders of common stock, with each holder entitled to the number of votes equal to the number of shares of common stock into which his or her shares of Series A Convertible Preferred Stock could be converted.
Dividend Rights. There is no dividend rate on the Series A Convertible Preferred Stock; however, the Company will pay holders of Series A Convertible Preferred Stock any dividend it declares with respect to the common stock on an as converted basis.
Conversion. The holders of Series A Convertible Preferred Stock have the right to convert such shares, at any time, into shares of common stock. The current conversion rate of the Series A Convertible Preferred Stock is 1-for-4, subject to further adjustment for certain corporate events, including stock splits, stock dividends, and recapitalizations. The Series A Convertible Preferred Stock automatically convert into common stock at the then applicable conversion rate upon the closing of an initial public offering or the consent of holders of a majority of the outstanding shares of the Series A Convertible Preferred Stock.
Liquidation. In the event of the liquidation, dissolution or winding-up of the Company, the holders of Series A Convertible Preferred Stock would be entitled to receive $1.00 per share before any liquidation distributions may be paid to the holders of the common stock.
Redemption. Shares of Series A Convertible Preferred Stock are not redeemable by the Company.
Registration Rights Agreement—The Company has an agreement with many of its current stockholders pursuant to which the Company has granted those stockholders certain registration rights. The stockholders who are parties to the agreement generally have two demand registration rights, which rights become effective as of the date that is six months after the Company’s initial public offering (as such these registration rights are contingent upon the successful completion of an initial public offering). A requisite percentage of holders is required to exercise a demand registration right, and certain other restrictions apply. Stockholders also have the right to participate on a “piggyback basis” in certain registrations by the Company under the Securities Act of 1933, subject to certain restrictions, including underwriter holdbacks.
F-21
SURGIVISION, INC.
Notes to Financial Statements
Years Ended December 31, 2009, 2008 and 2007 and the
Unaudited Three Month Periods Ended March 31, 2010 and 2009
Note 8 – Stockholders’ Equity – (continued)
Stock Incentive Plans—At March 31, 2010, the Company has two share-based compensation plans (the “2007 Plan” and the “1998 Plan”, and referred to collectively as the “Plans”). The 1998 Plan provides for the granting of qualified incentive and non-qualified stock options to employees, directors, consultants and advisors. The 2007 Plan provides for the granting of qualified incentive and non-qualified stock options, stock appreciation rights, restricted stock awards, restricted stock units, and other stock-based awards to employees, directors, consultants and advisors. Awards may be subject to a vesting schedule as set forth in each individual award agreement. The Company terminated the 1998 Plan, effective June 24, 2008, with respect to future grants such that no new options may be awarded under the 1998 Plan on or after June 24, 2008. The maximum shares of common stock which can be issued under the 2007 Plan is 625,000.
Activity with respect to the stock options is summarized as follows (no options were granted during the three months ended March 31, 2010):
| Options Outstanding |
Options Exercisable |
Range of Exercise Price |
Weighted- average Exercise price per share |
Intrinsic Value | ||||||||||
| Balance at January 1, 2007 |
303,750 | $ | 0.88 - 24.00 | $ | 1.72 | $ | 320,000 | |||||||
| Options exercisable at January 1, 2007 |
303,750 | 0.88 - 24.00 | 1.72 | 320,000 | ||||||||||
| Options granted |
147,500 | 3.20 | 3.20 | |||||||||||
| Balance at December 31, 2007 |
451,250 | 0.88 - 24.00 | 2.20 | 331,500 | ||||||||||
| Options exercisable at December 31, 2007 |
361,250 | 0.88 - 24.00 | 1.96 | 331,500 | ||||||||||
| Options granted |
154,875 | 6.04 - 9.64 | 7.84 | |||||||||||
| Options cancelled or forfeited |
(6,250 | ) | 6.00 | 6.00 | ||||||||||
| Outstanding at December 31, 2008 |
599,875 | 0.88 - 24.00 | 3.64 | 3,742,700 | ||||||||||
| Options exercisable at December 31, 2008 |
432,082 | 0.88 - 24.00 | 2.70 | 3,133,667 | ||||||||||
| Options granted |
93,402 | 9.64 | 9.64 | |||||||||||
| Options exercised |
(3,333 | ) | 3.20 | 3.20 | ||||||||||
| Options cancelled or forfeited |
(20,167 | ) | 1.64 - 20.00 | 9.60 | ||||||||||
| Outstanding at December 31, 2009 |
669,777 | 0.88 - 24.00 | 4.28 | 3,694,400 | ||||||||||
| Options exercisable at December 31, 2009 |
483,364 | 0.88 - 24.00 | 2.78 | 3,424,333 | ||||||||||
| Options cancelled or forfeited |
(2,500 | ) | 24.00 | 24.00 | ||||||||||
| Outstanding at March 31, 2010 |
667,277 | 0.88 - 24.00 | 4.20 | 3,694,400 | ||||||||||
| Options exercisable at March 31, 2010 |
481,697 | 0.88 - 24.00 | 2.68 | 3,424,333 | ||||||||||
| (1) | All Options granted during the year ended December 31, 2009 were granted with an exercise price of $9.64 per share, which was deemed to be the fair market value on the date of grant. |
F-22
SURGIVISION, INC.
Notes to Financial Statements
Years Ended December 31, 2009, 2008 and 2007 and the
Unaudited Three Month Periods Ended March 31, 2010 and 2009
Note 8 – Stockholders’ Equity – (continued)
The following table summarizes information about stock options at March 31, 2010:
| Options Outstanding | Options Exercisable | |||||||||||
| Range of Exercise Prices |
Number Outstanding |
Weighted - Average Remaining Contractual Life |
Weighted - Average Exercise Price |
Number Exercisable |
Weighted - Average Exercise Price | |||||||
| $ 0.88 - 3.20 |
427,500 | 5.05 | $ | 1.64 | 414,167 | $ | 1.59 | |||||
| 6.04 - 9.64 |
234,777 | 7.05 | 8.46 | 62,530 | 8.17 | |||||||
| 24.00 |
5,000 | 1.26 | 24.00 | 7,500 | 24.00 | |||||||
| 667,277 | 5.73 | 4.21 | 481,697 | 2.68 | ||||||||
The following table summarizes information about stock options at December 31, 2009:
| Options Outstanding | Options Exercisable | |||||||||||
| Range of Exercise Prices |
Number Outstanding |
Weighted - Average Remaining Contractual Life |
Weighted - Average Exercise Price |
Number Exercisable |
Weighted - Average Exercise Price | |||||||
| $ 0.88 - 3.20 |
427,500 | 5.30 | $ | 1.64 | 414,617 | $ | 1.59 | |||||
| 6.04 - 9.64 |
234,777 | 7.30 | 8.46 | 61,697 | 8.15 | |||||||
| 24.00 |
7,500 | 1.00 | 24.00 | 7,500 | 24.00 | |||||||
| 669,777 | 5.95 | 4.28 | 483,364 | 2.78 | ||||||||
The following table summarizes information about stock options at December 31, 2008:
| Options Outstanding | Options Exercisable | |||||||||||
| Range of Exercise Prices |
Number Outstanding |
Weighted - Average Remaining Contractual Life |
Weighted - Average Exercise Price |
Number Exercisable |
Weighted - Average Exercise Price | |||||||
| $ 0.88 - 3.20 |
435,000 | 6.33 | $ | 1.67 | 383,333 | $ | 1.47 | |||||
| 6.04 - 9.64 |
154,875 | 9.56 | 7.86 | 38,750 | 9.64 | |||||||
| 20.00 - 24.00 |
10,000 | 1.50 | 23.00 | 10,000 | 23.00 | |||||||
| 599,875 | 7.09 | 3.62 | 432,083 | 2.70 | ||||||||
F-23
SURGIVISION, INC.
Notes to Financial Statements
Years Ended December 31, 2009, 2008 and 2007 and the
Unaudited Three Month Periods Ended March 31, 2010 and 2009
Note 8 – Stockholders’ Equity – (continued)
The weighted-average grant date fair value of options granted during the years ended December 31, 2009 and 2008 are $2.83 and $2.32, respectively. A summary of the status of the Company’s nonvested stock options during the three months ended March 31, 2010 and the years ended December 31, 2009, 2008, and 2007 is presented below:
| Nonvested Stock Options |
Shares | Weighted - Average Grant Date Fair Value | ||||
| Nonvested January 1, 2007 |
— | $ | — | |||
| Granted |
90,000 | 0.40 | ||||
| Nonvested December 31, 2007 |
90,000 | 0.40 | ||||
| Granted |
116,125 | 2.23 | ||||
| Vested |
(38,333 | ) | 0.40 | |||
| Nonvested December 31, 2008 |
167,792 | 1.67 | ||||
| Granted |
93,402 | 2.83 | ||||
| Forfeited |
(7,250 | ) | 2.84 | |||
| Vested |
(67,531 | ) | 1.11 | |||
| Nonvested December 31, 2009 |
186,413 | 2.41 | ||||
| Vested |
833 | 2.68 | ||||
| Nonvested March 31, 2010 |
178,080 | 2.41 | ||||
As of March 31, 2010 there was a total of approximately $343,000 of unrecognized compensation cost related to nonvested share-based compensation arrangements granted under the Plans. That cost is expected to be recognized over a weighted-average period of approximately 2.75 years.
The assumptions used in calculating the fair value of options using the Black-Scholes option-pricing model are set forth in the following table:
| Year Ended December 31, | ||||||
| 2009 | 2008 | 2007 | ||||
| Dividend yield |
0% | 0% | 0% | |||
| Expected Volatility |
23.45% to 38.28% | 24.45% to 26.44% | 27.67% to 29.13% | |||
| Risk free Interest rates |
1.48% to 2.43% | 2.56% to 3.03% | 4.50% to 5.06% | |||
| Expected lives |
3.25 to 5.75 years | 5 to 5.75 years | 5 to 5.75 years | |||
Warrants—Warrants have been issued for terms of up to five years.
F-24
SURGIVISION, INC.
Notes to Financial Statements
Years Ended December 31, 2009, 2008 and 2007 and the
Unaudited Three Month Periods Ended March 31, 2010 and 2009
Note 8 – Stockholders’ Equity – (continued)
Common Stock warrants issued, expired, and outstanding during the three months ended March 31, 2010 and the years ended December 31, 2009, 2008 and 2007 are as follows:
| Number | Weighted Average Exercise Price per Share | |||||
| Warrants outstanding at January 1, 2007 |
410,542 | $ | 3.48 | |||
| Warrants issued during the year ended December 31, 2007 |
417,960 | 0.04 | ||||
| Warrants outstanding at December 31, 2008 and 2007 |
828,502 | 1.74 | ||||
| Warrants expired during the year ended December 31, 2009 |
(417,960 | ) | 0.04 | |||
| Warrants outstanding at December 31, 2009 |
410,542 | 3.48 | ||||
| Warrants issued during the three months ended March 31, 2010 |
25,444 | 8.00 | ||||
| Warrants outstanding at March 31, 2010 |
435,986 | $ | 3.74 | |||
The assumptions used in calculating the fair value of warrants utilizing the Black-Scholes pricing model are as follows:
| Warrants issued during | ||||||
| 2010 | 2007 | |||||
| Dividend yield |
0 | % | 0 | % | ||
| Expected volatility |
45.98 | % | 20 | % | ||
| Risk free rates |
2.6 | % | 4.91 | % | ||
| Expected term |
5 years | 1 year | ||||
Stock Transactions with Related Parties –
| • | During January 2009, the Company loaned $500,000 under an 8% note receivable to a stockholder with an original maturity date in July 2010. The note was collateralized by 125,000 shares of the Company’s common stock owned by the stockholder. In addition, during January 2009, the Company purchased 125,000 shares of the Company’s common stock from that same stockholder for $500,000 in cash (accounted for as a treasury stock purchase). During December 2009, the Company purchased 134,178 additional shares of the Company’s common stock from this stockholder in exchange for cancellation of the aforementioned $500,000 note receivable plus $36,712 of accrued interest thereon. |
| • | The Company had a note receivable from its Chief Executive Officer (“CEO”) related to the sale of common stock. The note bears interest at 4.5%. Interest income related to this note was approximately $21,100 for the year ended December 31, 2009 and approximately $21,700 for each of the two years ended December 31, 2008 and 2007. On December 22, 2009, the Company purchased 66,652 shares of common stock from the CEO, for an aggregate purchase price of $642,525. The Company paid a portion of the aggregate purchase price ($594,687) by cancelling the aforementioned promissory note plus accrued interest, with the remainder paid in cash. Also, on December 22, 2009, the Company issued to the CEO options to purchase 66,652 shares of its common stock at an exercise price of $9.64 per share. |
F-25
SURGIVISION, INC.
Notes to Financial Statements
Years Ended December 31, 2009, 2008 and 2007 and the
Unaudited Three Month Periods Ended March 31, 2010 and 2009
Note 9 – Income Taxes
The Company recorded federal income tax expense of $49,250 for the year ended December 31, 2009 related to alternative minimum tax due which cannot be offset by net operating loss carryforwards. The Company had no income tax expense or benefit for the years ended 2008 and 2007. As the Company has incurred net operating losses, the Company has recognized valuation allowances for all deferred tax assets. The tax effect of temporary differences and net operating losses that give rise to components of deferred tax assets and liabilities consist of the following:
| March
31, 2010 |
December 31, | |||||||||||||||
| 2009 | 2008 | 2007 | ||||||||||||||
| Deferred tax assets (liabilities): |
||||||||||||||||
| Furniture, software and equipment |
$ | (200,384 | ) | $ | (202,296 | ) | $ | (144,776 | ) | $ | (4,886 | ) | ||||
| Other |
28,400 | 60,139 | — | — | ||||||||||||
| Deferred revenue |
3,244,204 | 3,207,620 | (8,139 | ) | — | |||||||||||
| Accrued expenses |
377,229 | 439,965 | 110,891 | 3,778 | ||||||||||||
| Net operating loss carryforward |
12,435,688 | 11,591,052 | 12,491,917 | 10,690,528 | ||||||||||||
| 15,885,138 | 15,096,480 | 12,449,893 | 10,689,420 | |||||||||||||
| Less: valuation allowance |
(15,885,138 | ) | (15,096,480 | ) | (12,449,893 | ) | (10,689,420 | ) | ||||||||
| $ | — | $ | — | $ | — | $ | — | |||||||||
The Company has a cumulative federal net operating loss of approximately $33,000,000 as of March 31, 2010. Under Section 382 and 383 of the Internal Revenue Code, if an ownership change occurs with respect to a “loss corporation”, as defined, there are annual limitations on the amount of the net operating loss and other deductions which are available to the Company. The Company has not determined whether such ownership change has occurred. However, given the equity transactions in which the Company has engaged, the Company believes that the use of the net operating losses shown as deferred tax assets will be significantly limited.
Note 10 – Commitments
Leases—The Company leases office space in Maryland, California and Tennessee under non-cancellable operating leases. Leases expire in 2011, 2012 and 2014.
Future minimum lease payments under non-cancellable operating leases are as follows:
| Years ending December 31, | |||
| 2010 |
$ | 145,331 | |
| 2011 |
171,545 | ||
| 2012 |
124,018 | ||
| 2013 |
62,272 | ||
| 2014 |
58,399 | ||
| Total minimum payments |
$ | 561,565 | |
F-26
SURGIVISION, INC.
Notes to Financial Statements
Years Ended December 31, 2009, 2008 and 2007 and the
Unaudited Three Month Periods Ended March 31, 2010 and 2009
Note 10 – Commitments – (continued)
Co-Development Agreement—The Company has entered into a co-development agreement whereby the Company is required to pay up to approximately $2,476,000 in milestone-based payments for software development to be used in conjunction with products being developed by the Company. The software, upon completion, will be owned by the co-developer and sold through licenses. The co-developer will pay the Company a fixed amount per license sold by the co-developer until the Company recoups its investment in the software. The Company’s remaining milestone-based payments under the co-development agreement at March 31, 2010 totaled approximately $2,176,000, which is expected to be paid in installments through September 2011.
Shared Research Agreements—The Company has entered into research agreements with certain universities whereby the Company has committed to pay certain research-related expenses. As of March 31, 2010 the Company is committed to pay additional amounts aggregating approximately $920,000, which will be payable at various dates through January 2011. In addition, the Company has agreed to provide in kind equipment and services over a two year period once the equipment is installed.
Software License Agreement—The Company is obligated under a master services and license agreement to purchase a minimum number of licenses for software code that is incorporated in the Company’s ClearPoint system software. The minimum future purchase obligation is $525,000 for each of the contract years ending July 2010, 2011 and 2012. The cost of each license will be charged to cost of sales as each ClearPoint system is sold, which sales are subject prior to FDA clearance.
Note 11 – Subsequent Events
Litigation
On April 22, 2010, SurgiVision Consultants, Inc. and Guy M. Kezirian filed a lawsuit against the Company in the United States District Court, Central District of California, alleging trademark infringement, unfair competition, trademark dilution and violation of the Anti-Cybersquatting Protection Act, all relating to the Company’s use of its SURGI-VISION and SURGIVISION trademarks and the Company’s www.surgivision.com domain name. The plaintiffs are seeking unspecified monetary damages and injunctive relief. This action is at the very preliminary stage. The Company believes it has strong defenses to the allegations, and intends to vigorously defend itself in the lawsuit to protect its trademark rights. Due to the preliminary stage of the proceedings, management is unable to determine the financial impact, if any, of the ultimate outcome of this matter. No liability, which might result from this matter, if any, has been recorded in the Company’s financial statements.
Reverse Stock Split
On April 23, 2010, the Company’s stockholders approved an amendment to the Company’s certificate of incorporation giving the Board of Directors (the “Board”) the discretion to effect a reverse split of the shares of the Company’s common stock (the “Reverse Split”). On June 14, 2010, a duly authorized committee of the Board approved a 1-for-4 Reverse Split to be effected prior to the effective date of the Company’s initial public offering. The Reverse Split will not change the number of authorized shares or the par value of the Company’s common stock. In connection with the Reverse Split, the Company’s Series A Convertible Preferred Stock, outstanding convertible notes and outstanding options and warrants will be adjusted so that the number of shares
F-27
SURGIVISION, INC.
Notes to Financial Statements
Years Ended December 31, 2009, 2008 and 2007 and the
Unaudited Three Month Periods Ended March 31, 2010 and 2009
Note 11 – Subsequent Events – (continued)
of common stock issuable upon their conversion or exercise will be decreased proportionately, and the conversion or exercise price will be increased proportionately. On July 13, 2010, the Company filed the amendment to the Company’s certificate of incorporation, consummating the Reverse Split. Accordingly, all share and per share amounts for all periods presented in these financial statements and notes thereto, have been adjusted retroactively, where applicable, to reflect this Reverse Split.
New Stock Incentive Plan
On March 23, 2010, the Board approved the 2010 Incentive Compensation Plan (the “2010 Plan”) and recommended it to the stockholders. The 2010 Plan was approved by a vote of the stockholders on April 23, 2010. The principal purpose of the 2010 Plan is to attract, retain and motivate selected employees, consultants and directors through the granting of stock-based compensation awards and cash-based performance awards. The 2010 Plan is also designed to permit the Company to make cash-based awards and equity-based awards intended to quality as “performance-based compensation” under 162(m) of the Internal Revenue Code. A total of 1,250,000 shares of the Company’s common stock have been reserved for issuance under the 2010 Plan. Upon adoption of the 2010 Plan, the Company ceased making awards under its 2007 Plan. In connection with the Company’s initial public offering, the Company intends to (i) grant options under the 2010 Plan to purchase 541,000 shares of the Company’s common stock with exercise prices equal to the initial public offering price, and (ii) issue 30,000 shares of the Company’s common stock under the 2010 Plan, assuming an initial public offering price of $10.00 per share and taking into account the 1-for-4 Reverse Split described above.
Cardiac EP Business Participation Plan
On June 2, 2010, the Board approved the Cardiac EP Business Participation Plan to enable the Company to provide a key product development advisor and consultant with financial rewards in the event the Company sells its business operations relating to catheter-based MRI-guided cardiac ablation to treat cardiac arrhythmias, which the Company refers to as its cardiac EP operations. In the event the Company sells its cardiac EP operations, whether on a stand-alone basis or as part of the sale of the Company, the plan participant will receive a payment under the plan equal to (i) the transaction value paid for or allocated to the cardiac EP operations in the sale, multiplied by (ii) the participant’s participation interest under the plan at the time of the sale. The Plan participant was initially awarded a participation interest of 6.6%. However, that percentage interest will be equitably reduced from time to time to take into account any equity financing transactions, including the Company’s initial public offering, in which the Company issues shares of its common stock or securities convertible into shares of its common stock in exchange for cash proceeds. The plan will terminate on June 2, 2025.
Key Personnel Incentive Program
On June 2, 2010, the Board approved the amendment of the Company’s Key Personnel Incentive Program, which provides a key employee and consultant with the opportunity to receive incentive bonus payments based on the performance of future services to the Company or upon a consummation of a transaction involving the sale of the Company. In the event of a sale transaction, each participant will receive a bonus payment under the program if the participant continues to provide services to the Company as its employee or consultant as of the date of the transaction. Until the occurrence of a sale transaction, each participant will be entitled to receive semi-annual service bonuses beginning on June 30, 2012 and continuing through December 31, 2015, if the participant continues to provide services to the Company as its employee or consultant as of the respective scheduled payment dates. Pursuant to their awards, the two participants would receive service bonuses totaling up to
F-28
SURGIVISION, INC.
Notes to Financial Statements
Years Ended December 31, 2009, 2008 and 2007 and the
Unaudited Three Month Periods Ended March 31, 2010 and 2009
Note 11 – Subsequent Events – (continued)
$1,700,000 and $1,000,000, respectively, payable in eight equal semi-annual installments. If the participant’s employment or consultancy is (i) terminated due to the participant’s death or disability, or (ii) involuntarily terminated by the Company other than for cause, then the participant will be deemed vested, as of the termination date, in all future scheduled service bonus payments, and the Company will be required to pay that aggregate amount no later than March 15 of the year following the year in which the termination occurred. If the participant’s employment or consultancy is involuntarily terminated by the Company for cause, or if the participant voluntarily terminates his employment of consultancy, the participant thereafter will not be entitled to any payments under the program. The program will terminate on the earlier of December 31, 2015 or the occurrence of a transaction involving the sale of the Company.
Merger of Subsidiary
On June 11, 2010, the Company’s sole subsidiary, Cardiac EP Sub, Inc., was merged with and into the Company.
FDA Clearance
On June 16, 2010, the Company received regulatory clearance from the FDA to market its ClearPoint system in the United States for general neurological procedures. The Company will market its ClearPoint system to provide guidance for the placement and operation of instruments or devices during the planning and operation of neurological procedures within the MRI environment and in conjunction with MR imaging. The Company anticipates that it will begin selling its ClearPoint system products in the first half of the third quarter of 2010.
F-29


2,500,000 Shares
SurgiVision, Inc.
Common Stock
Prospectus
| Canaccord Genuity |
Rodman & Renshaw, LLC |
, 2010
PART II
INFORMATION NOT REQUIRED IN PROSPECTUS
| Item 13. | Other Expenses of Issuance and Distribution |
The following table sets forth the costs and expenses to be paid by us in connection with the sale of the shares of common stock being registered hereby. All amounts are estimates except for the SEC registration fee, the FINRA filing fee and the Nasdaq Capital Market listing fee.
| Amount to be Paid | |||
| SEC registration fee |
$ | 3,075 | |
| FINRA filing fee |
3,500 | ||
| Nasdaq Capital Market listing fee |
50,000 | ||
| Printing and engraving expenses |
200,000 | ||
| Blue sky qualification fees and expenses |
15,000 | ||
| Accounting fees and expenses |
200,000 | ||
| Legal fees and expenses |
800,000 | ||
| Transfer agent and registrar fees |
3,500 | ||
| Miscellaneous expenses |
650,000 | ||
| Total |
$ | 1,925,075 | |
| Item 14. | Indemnification of Directors and Officers |
Our certificate of incorporation, which will become effective upon the completion of this offering, contains provisions permitted under Delaware law relating to the liability of directors. These provisions eliminate a director’s personal liability for monetary damages resulting from a breach of fiduciary duty, except in circumstances involving wrongful acts, such as any:
| • | breach of the director’s duty of loyalty to us or our stockholders; |
| • | act or omission not in good faith or that involves intentional misconduct or a knowing violation of the law; |
| • | act related to unlawful stock repurchases, redemptions or other distribution or payments of dividends; or |
| • | transaction from which the director derived an improper personal benefit. |
These provisions do not limit or eliminate our rights or any stockholder’s rights to seek non-monetary relief, such as an injunction or rescission, in the event of a breach of director’s fiduciary duty. These provisions will not alter a director’s liability under federal securities laws.
As permitted by Section 145 of the Delaware General Corporation Law, our bylaws, which will become effective upon the closing of this offering, require us to indemnify our directors and executive officers to the fullest extent not prohibited by the Delaware law. We may limit the extent of such indemnification by individual contracts with our directors and executive officers. Further, we may decline to indemnify any director or executive officer in connection with any proceeding initiated by such person or any proceeding by such person against us or our directors, officers, employees or other agents, unless such indemnification is expressly required to be made by law or the proceeding was authorized by our Board of Directors.
We have entered into indemnity agreements with each of our current directors and certain of our executive officers to give such directors and officers additional contractual assurances regarding the scope of the indemnification set forth in our certificate of incorporation and bylaws and to provide additional procedural
II-1
protections. At present, there is no pending litigation or proceeding involving any of our directors, officers or employees for which indemnification is sought, nor are we aware of any threatened litigation that may result in claims for indemnification.
We have the power to indemnify our other officers, employees and other agents, as permitted by Delaware law, but we are not required to do so.
The Registrant maintains a directors’ and officers’ insurance and registrant reimbursement policy. The policy insures directors and officers against unindemnified losses arising from certain wrongful acts in their capacities as directors and officers and reimburses the registrant for those losses for which the registrant has lawfully indemnified the directors and officers. The policy contains various exclusions, none of which apply to this offering.
Reference is made to the following documents filed as exhibits to this registration statement regarding relevant indemnification provisions described above and elsewhere herein:
| Exhibit Document |
Number | |
| Form of Underwriting Agreement |
1.1 | |
| Form of Amended and Restated Certificate of Incorporation |
3.3 | |
| Form of Amended and Restated Bylaws |
3.4 | |
| Third Amended and Restated Investor Rights’ Agreement dated September 20, 2006 |
3.5 | |
| First Amended and Restated Stockholders’ Agreement dated April 30, 2004 |
3.6 | |
| Form of Indemnification Agreement |
10.8 |
| Item 15. | Recent Sales of Unregistered Securities |
The following sets forth information regarding all unregistered securities sold since December 31, 2006:
1. We have granted stock options to purchase an aggregate of 329,125 shares of common stock to employees, consultants and directors under our 2007 Stock Incentive Plan, which makes available an aggregate of 625,000 shares of common stock. Stock options to purchase 1,284,167 shares of our common stock remain outstanding. The issuance of these options was exempt from registration under Section 4(2) of the Securities Act, as a sale not involving a public offering, or pursuant to Rule 701 under the Securities Act.
2. On December 22, 2009, we issued to Mr. Jenkins an option to purchase 66,652 shares of our common stock at an exercise price of $9.64 per share. The issuance of this option was exempt from registration under 4(2) of the Securities Act, as a sale not involving a public offering.
3. Between January 2006 and August 2007, Boston Scientific, one of our 5% common stockholders and the employer of one of our directors, loaned us $1,500,000 in six equal quarterly installments pursuant to a convertible promissory note. This note became payable on June 30, 2008, at which time Boston Scientific converted the note into 417,960 shares of our common stock and a warrant for 417,960 shares of our common stock, which warrant has since expired.
4. In November and December of 2006, we issued and sold an aggregate of 7,965,000 shares of our Series A Convertible Preferred Stock to 48 accredited investors at $1.00 per share, for an aggregate offering price of $7,965,000. Upon completion of this offering, these shares of preferred stock will automatically convert into shares of common stock. In connection with this Series A Preferred Stock offering, we engaged Gilford Securities Incorporated to serve as a placement agent. As placement agent, Gilford Securities Incorporated received a cash fee of approximately $475,000 and a warrant exercisable for 141,500 shares of common stock at an exercise price of $4.00 per share.
II-2
5. On November 2, 2006, we issued a warrant exercisable for 12,500 shares of common stock at an exercise price of $4.00 per share. This warrant was exempt from registration under Section 4(2) of the Securities Act, as a sale not involving a public offering.
6. During 2009, Boston Scientific loaned us $3,500,000 pursuant to the terms of three convertible promissory notes. Interest on the loans accrues at 10% per annum and compounds annually. The Boston Scientific loans are secured by a first priority security interest in all of our assets. Each loan matures on the second anniversary of the date on which the funds were advanced. In addition, we will be required to prepay all or a portion of loans upon the consummation of any qualified financing, which is any equity financing in which shares of our preferred stock are issued in exchange for cash proceeds. Upon consummation of a qualified financing from Medtronic, Inc., St. Jude Medical, Inc., or Johnson & Johnson, or any of their respective subsidiaries or affiliates, up to 100% of the cash proceeds from such qualified financing must be used to prepay the outstanding principal of the loans and accrued interest thereon. Upon consummation of a qualified financing from any other investor, up to 25% of the cash proceeds from such qualified financing shall be applied by us to prepay the outstanding principal of the loans and accrued interest thereon. We can repay each loan at anytime prior to its respective maturity date. At the option of Boston Scientific, these loans are convertible into one share of our common stock for every $8.00 of principal and interest outstanding at the time of conversion. To the extent that Boston Scientific has not exercised its conversion right prior to the completion of this offering, Boston Scientific will no longer have the right to convert the notes into shares of stock.
7. On December 21, 2007, we made a restricted stock award to one of our consultants for 500 shares of common stock. This award was made under our 2007 Stock Incentive Plan. This restricted stock award was exempt from registration under Section 4(2) of the Securities Act, as a sale not involving a public offering, or pursuant to Rule 701 under the Securities Act.
8. In March 2010, we issued 10% senior unsecured convertible notes, or the bridge notes, in the aggregate principal amount of approximately $4.1 million to 50 accredited investors in a private placement, or the bridge financing. Upon consummation of this offering, the bridge notes will automatically convert into shares of our common stock upon the closing of this offering at the lesser of $8.00 per share or 80% of the offering price in this offering, subject to a $4.00 per share floor conversion price. In addition, subject to prior maturity, prepayment and/or certain adjustments, holders of the bridge notes may convert the outstanding principal amount of their bridge notes into shares of our common stock at any time, based on a conversion price of $8.00 per share. The bridge notes mature two years from the date of issuance, unless earlier converted, and accrue interest at the rate of 10% per annum. All accrued interest will be paid in cash and will not be converted into shares of our common stock. In connection with the bridge financing, we engaged Gilford Securities Incorporated to serve as a placement agent. As placement agent, Gilford Securities Incorporated received a cash fee of approximately $285,000 and a warrant exercisable for 25,444 shares of our common stock at a price equal to the lesser of $8.00 per share or 80% of the offering price in our initial public offering, subject to a $4.00 per share floor conversion price.
We claimed exemption from registration under the Securities Act for the sales and issuances of securities in the transactions described in paragraphs (3) through (8) by virtue of Section 4(2) of the Securities Act and/or Rule 506 of Regulation D. Such sales and issuances did not involve any public offering, were made without general solicitation or advertising and each purchaser was a sophisticated investor with access to all relevant information necessary to evaluate the investment and represented to us that the shares were being acquired for investment.
II-3
| Item 16. | Exhibits and Financial Statement Schedules |
(a) Exhibits
| Number |
Description | |
| 1.1** | Form of Underwriting Agreement(7) | |
| 3.1** | Amended and Restated Certificate of Incorporation of SurgiVision, Inc., as amended(1) | |
| 3.2** | Bylaws of SurgiVision, Inc., as amended(1) | |
| 3.3** | Form of Amended and Restated Certificate of Incorporation of SurgiVision, Inc. to be effective upon completion of this offering(5) | |
| 3.4** | Form of Amended and Restated Bylaws of SurgiVision, Inc. to become effective upon completion of this offering(5) | |
| 3.5** | Third Amended and Restated Investor Rights’ Agreement dated September 20, 2006, as amended(1) | |
| 3.6** | First Amended and Restated Stockholders’ Agreement dated April 30, 2004(1) | |
| 3.7** | Certificate of Designation, Preferences and Rights of Series A Convertible Preferred Stock of Surgi-Vision, Inc. filed with the State of Delaware on September 20, 2006(3) | |
| 4.1 | Reference is made to Exhibits 3.1, 3.2, 3.3 and 3.4 | |
| 4.2** | Specimen of Common Stock Certificate(5) | |
| 4.3** | Form of SurgiVision, Inc. 10% Senior Unsecured Convertible Note Due 2012(5) | |
| 4.4** | SurgiVision, Inc. Warrant to Purchase Common Stock, dated March 30, 2010, issued to Gilford Securities Incorporated(5) | |
| 5.1 | Opinion of Baker, Donelson, Bearman, Caldwell & Berkowitz, PC | |
| 10.1** | Surgi-Vision, Inc. 1998 Stock Option Plan(1) | |
| 10.2** | Surgi-Vision, Inc. 2007 Stock Incentive Plan(1) | |
| 10.3** | SurgiVision, Inc. Amended and Restated Key Personnel Incentive Program(7) | |
| 10.4** | 2010 Incentive Compensation Plan(5) | |
| 10.5** | 2010 Incentive Compensation Plan Form of Incentive Stock Option Agreement(7) | |
| 10.6** | 2010 Incentive Compensation Plan Form of Non-Qualified Stock Option Agreement(7) | |
| 10.7** | 2010 Incentive Compensation Plan Form of Non-Qualified Stock Option Agreement for Non-Employee Directors(7) | |
| 10.8** | Form of Indemnification Agreement(5) | |
| 10.9**† | License Agreement by and between SurgiVision, Inc. and The Johns Hopkins University entered into on or around June 20, 1998, as amended by that certain Amendment to License Agreement dated as of January 15, 2000, and as further amended by that certain Addendum to License Agreement entered into on or around December 7, 2004(7) | |
| 10.10**† | License Agreement by and between SurgiVision, Inc. and The Johns Hopkins University entered into on or around December 7, 2006(7) | |
| 10.11**† | Technology License Agreement dated as of December 30, 2005 by and between SurgiVision, Inc. and Boston Scientific Neuromodulation Corporation (formerly known as Advanced Bionics Corporation), as amended by that certain Omnibus Amendment dated June 30, 2007, and as further amended by that certain Omnibus Amendment #2 dated March 19, 2008(7) | |
| 10.12**† | System and Lead Development and Transfer Agreement dated as of December 30, 2005 by and between SurgiVision, Inc. and Boston Scientific Neuromodulation Corporation (formerly known as Advanced Bionics Corporation), as amended by that certain Amendment No. 1 dated May 31, 2006, as further amended by that certain Omnibus Amendment dated June 30, 2007, and as further amended by that certain Omnibus Amendment #2 dated March 19, 2008(7) | |
II-4
| Number |
Description | |
| 10.13**† | Technology License Agreement dated as of March 19, 2008 by and between SurgiVision, Inc. and Cardiac Pacemakers, Inc.(7) | |
| 10.14**† | Development Agreement dated as of March 19, 2008 by and between SurgiVision, Inc. and Cardiac Pacemakers, Inc.(6) | |
| 10.15**† | Cooperation and Development Agreement, dated as of May 4, 2009, by and between SurgiVision, Inc. and Siemens Aktiengesellschaft, Healthcare Sector(7) | |
| 10.16** | Consulting Agreement, effective as of May 1, 2009, by and between SurgiVision, Inc. and Dr. Paul Bottomley(4) | |
| 10.17** | Stock Purchase Agreement, dated December 22, 2009, by and between SurgiVision, Inc. and Kimble L. Jenkins(3) | |
| 10.18** | Non-Qualified Stock Option Agreement, dated December 22, 2009, by and between SurgiVision, Inc. and Kimble L. Jenkins(3) | |
| 10.19**† | Patent License Agreement – Nonexclusive entered into on or around April 27, 2009 by and between SurgiVision, Inc. and National Institutes of Health(7) | |
| 10.20**† | Master Services and Licensing Agreement dated as of July 20, 2007 by and between SurgiVision, Inc. and Cedara Software Corp. (7) | |
| 10.21**† | Exclusive License Agreement entered into on or around June 30, 2008 by and between SurgiVision, Inc. and The Johns Hopkins University(7) | |
| 10.22**† | Exclusive License Agreement entered into on or around June 30, 2008 by and between SurgiVision, Inc. and The Johns Hopkins University(7) | |
| 10.23**† | Exclusive License Agreement entered into on or around June 30, 2008 by and between SurgiVision, Inc. and The Johns Hopkins University(7) | |
| 10.24**† | Loan Agreement dated as of October 16, 2009 by and between SurgiVision, Inc. and Boston Scientific Corporation(2) | |
| 10.25**† | Patent Security Agreement dated as of October 16, 2009 by and between SurgiVision, Inc. and Boston Scientific Corporation(2) | |
| 10.26**† | Sponsored Research Agreement by and between SurgiVision, Inc. and the Regents of the University of California on behalf of its San Francisco campus entered into on or around August 24, 2007, as amended by that certain First Amendment to Sponsored Research Agreement dated December 1, 2008, as further amended by that certain Second Amendment to Sponsored Research Agreement dated May 1, 2009, as further amended by that certain Third Amendment to Sponsored Research Agreement dated November 2, 2009, as further amended by that certain Addendum to Sponsored Research Agreement dated February 4, 2010(6) | |
| 10.27**† | Research Agreement by and between SurgiVision, Inc. and The University of Utah entered into on or around July 2, 2007, as amended by that certain First Amendment to the Research Agreement entered into on or around January 8, 2008, as further amended by that certain Second Amendment to the Research Agreement dated April 24, 2009, as further amended by that certain Third Amendment to the Research Agreement dated May 1, 2009, as further amended by that certain Fourth Amendment to the Research Agreement entered into on or around February 25, 2010(6) | |
| 10.28** | Lease Agreement, dated as of April 21, 2008, by and between Shaw Investment Company, LLC and Surgi-Vision, Inc.(5) | |
| 10.29** | Separation Agreement, dated as of April 30, 2010, by and between John Thomas and SurgiVision, Inc.(7) | |
II-5
| Number |
Description | |
| 10.30** | SurgiVision, Inc. Cardiac EP Business Participation Plan(7) | |
| 10.31** | Cardiac EP Business Participation Plan Award Agreement, dated June 3, 2010, by and between SurgiVision, Inc. and Nassir F. Marrouche(7) | |
| 10.32** | Employment Agreement, dated as of June 3, 2010, by and between SurgiVision, Inc. and Kimble L. Jenkins (8) | |
| 10.33** | Employment Agreement, dated as of June 3, 2010, by and between SurgiVision, Inc. and Peter G. Piferi (8) | |
| 10.34** | Employment Agreement, dated as of June 3, 2010, by and between SurgiVision, Inc. and David W. Carlson (8) | |
| 10.35** | Employment Agreement, dated as of June 3, 2010, by and between SurgiVision, Inc. and Oscar L. Thomas (8) | |
| 10.36** | Employment Agreement, dated as of June 3, 2010, by and between SurgiVision, Inc. and John T. Keane (8) | |
| 10.37** | Employment Agreement, dated as of June 3, 2010, by and between SurgiVision, Inc. and Michael M. Moore(8) | |
| 10.38** | Employment Agreement, dated as of June 3, 2010, by and between SurgiVision, Inc. and Carol J. Barbre (8) | |
| 10.39** | Amended and Restated Key Personnel Incentive Award Agreement, dated June 2, 2010, by and between SurgiVision, Inc. and Paul A. Bottomley(7) | |
| 10.40** | Amended and Restated Key Personnel Incentive Award Agreement, dated June 2, 2010, by and between SurgiVision, Inc. and Paul A. Bottomley(7) | |
| 10.41** | Amended and Restated Key Personnel Incentive Award Agreement, dated June 2, 2010, by and between SurgiVision, Inc. and Parag V. Karmarkar(7) | |
| 10.42** | Form of Non-Competition Agreement(7) | |
| 10.43** | Stock Purchase and Loan Agreement, dated January 30, 2009, by and between SurgiVision, Inc. and DARA Biosciences, Inc.(8) | |
| 10.44** | Secured Promissory Note, dated January 30, 2009, issued by DARA Pharmaceuticals, Inc. to SurgiVision, Inc.(8) | |
| 10.45** | Stock Pledge Agreement, dated January 30, 2009, by and between SurgiVision, Inc. and DARA Pharmaceuticals, Inc.(8) | |
| 10.46** | Stockholder Agreement, dated January 30, 2009, by and between SurgiVision, Inc. and DARA Pharmaceuticals, Inc.(8) | |
| 10.47** | Stock Purchase Agreement, dated December 31, 2009, by and between SurgiVision, Inc. and DARA Pharmaceuticals, Inc.(8) | |
| 21** | List of Subsidiary (9) | |
| 23.1 | Consent of Cherry, Bekaert & Holland, L.L.P. | |
| 23.2** | Consent of Baker, Donelson, Bearman, Caldwell & Berkowitz, PC (included in Exhibit 5.1) | |
| 24.1** | Power of attorney. Reference is made to the signature page.(1) | |
| 24.2** | Power of Attorney(7) | |
II-6
| * | To be filed by amendment. |
| ** | Previously filed. |
| † | Confidential treatment requested under 17 C.F.R. §§200.80(b)(4) and 230.406. The confidential portions of this exhibit have been omitted and are marked accordingly. The confidential portions have been filed separately with the Securities and Exchange Commission pursuant to the confidential treatment request. |
| (1) | Previously filed with the Securities and Exchange Commission on December 23, 2009 on the Registrant’s Registration Statement on Form S-1 (SEC File No. 333-163957). |
| (2) | Previously filed with the Securities and Exchange Commission on February 9, 2010 on the Registrant’s Registration Statement on Form S-1/A (SEC File No. 333-163957). |
| (3) | Previously filed with the Securities and Exchange Commission on February 26, 2010 on the Registrant’s Registration Statement on Form S-1/A (SEC File No. 333-163957). |
| (4) | Previously filed with the Securities and Exchange Commission on April 6, 2010 on the Registrant’s Registration Statement on Form S-1/A (SEC File No. 333-163957). |
| (5) | Previously filed with the Securities and Exchange Commission on May 7, 2010 on the Registrant’s Registration Statement on Form S-1/A (SEC File No. 333-163957). |
| (6) | Previously filed with the Securities and Exchange Commission on May 13, 2010 on the Registrant’s Registration Statement on Form S-1/A (SEC File No. 333-163957). |
| (7) | Previously filed with the Securities and Exchange Commission on June 4, 2010 on the Registrant’s Registration Statement on Form S-1/A (SEC File No. 333-163957). |
| (8) | Previously filed with the Securities and Exchange Commission on June 25, 2010 on the Registrant’s Registration Statement on Form S-1/A (SEC File No. 333-163957). |
| (9) | Previously filed with the Securities and Exchange Commission on July 2, 2010 on the Registrant’s Registration Statement on Form S-1/A (SEC File No. 333-163957). |
| Item 17. | Undertakings |
The undersigned Registrant hereby undertakes to provide to the underwriters at the closing specified in the Underwriting Agreement, certificates in such denominations and registered in such names as required by the underwriters to permit prompt delivery to each purchaser.
Insofar as indemnification for liabilities arising under the Securities Act may be permitted to directors, officers and controlling persons of the Registrant pursuant to the foregoing provisions, or otherwise, the Registrant has been advised that in the opinion of the Securities and Exchange Commission such indemnification is against public policy as expressed in the Securities Act and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the Registrant of expenses incurred or paid by a director, officer or controlling person of the Registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the Registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Securities Act and will be governed by the final adjudication of such issue.
The undersigned Registrant hereby undertakes that:
(1) For purposes of determining any liability under the Securities Act, the information omitted from the form of prospectus filed as part of this registration statement in reliance upon Rule 430A and contained in a form of prospectus filed by the Registrant pursuant to Rule 424(b)(1) or (4) or 497(h) under the Securities Act shall be deemed to be part of this registration statement as of the time it was declared effective.
(2) For the purpose of determining any liability under the Securities Act, each post-effective amendment that contains a form of prospectus shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
II-7
That, for the purpose of determining liability under the Securities Act of 1933 to any purchaser, if the Registrant is subject to Rule 430C, each prospectus filed pursuant to Rule 424(b) as part of a registration statement relating to an offering, other than registration statements relying on Rule 430B or other than prospectuses filed in reliance on Rule 430A, shall be deemed to be part of and included in the Registration Statement as of the date it is first used after effectiveness. Provided, however , that no statement made in a registration statement or prospectus that is part of the Registration Statement or made in a document incorporated or deemed incorporated by referenced into the Registration Statement or prospectus that is part of the Registration Statement will, as to a purchaser with a time of contract of sale prior to such first use, supersede or modify any statement that was made in the Registration Statement or prospectus that was part of the Registration Statement or made in any such document immediately prior to such date of first use.
That, for the purpose of determining liability of the Registrant under the Securities Act of 1933 to any purchaser in the initial distribution of the securities, the undersigned Registrant undertakes that in a primary offering of securities of the undersigned Registrant pursuant to this Registration Statement, regardless of the underwriting method used to sell the securities to the purchaser, if the securities are offered or sold to such purchaser by means of any of the following communications, the undersigned Registrant will be a seller to the purchaser and will be considered to offer or sell such securities to such purchaser:
(i) Any preliminary prospectus or prospectus of the undersigned Registrant relating to the offering required to be filed pursuant to Rule 424;
(ii) Any free writing prospectus relating to the offering prepared by or on behalf of the undersigned Registrant or used or referred to by the undersigned Registrant;
(iii) The portion of any other free writing prospectus relating to the offering containing material information about the undersigned Registrant or its securities provided by or on behalf of the undersigned Registrant; and
(iv) Any other communication that is an offer in the offering made by the undersigned Registrant to the purchaser.
II-8
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, as amended, SurgiVision, Inc. has duly caused this Amendment No. 11 to the Registration Statement on Form S-1 to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Memphis, State of Tennessee, on the 21st day of July, 2010.
| SurgiVision, Inc. | ||
| By: | /s/ KIMBLE L. JENKINS | |
| Kimble L. Jenkins | ||
| Chief Executive Officer (principal executive officer) | ||
POWER OF ATTORNEY
Pursuant to the requirements of the Securities Act of 1933, this Amendment No. 11 to the Registration Statement has been signed by the following persons in the capacities and on the dates indicated.
| Signature |
Title |
Date | ||
| /s/ KIMBLE L. JENKINS Kimble L. Jenkins |
Chief Executive Officer and Director (principal executive officer) |
July 21, 2010 | ||
| /s/ DAVID W. CARLSON David W. Carlson |
Chief Financial Officer (principal accounting officer and principal financial officer) |
July 21, 2010 | ||
| * John C. Thomas, Jr. |
Director |
July 21, 2010 | ||
| * Paul A. Bottomley |
Director |
July 21, 2010 | ||
| * Lenox D. Baker |
Director |
July 21, 2010 | ||
| * Charles E. Koob |
Director |
July 21, 2010 | ||
| * Wendelin C. Maners |
Director |
July 21, 2010 | ||
| * John N. Spencer |
Director |
July 21, 2010 | ||
| /S/ JAMES K. MALERNEE, JR. James K. Malernee, Jr. |
Director |
July 21, 2010 | ||
| * Michael A. Pietrangelo |
Director |
July 21, 2010 | ||
| *By |
/s/ KIMBLE L. JENKINS Kimble L. Jenkins |
Attorney-in-Fact |
July 21, 2010 | |||
II-9
EXHIBIT INDEX
| Number |
Description | |
| 1.1** | Form of Underwriting Agreement(7) | |
| 3.1** | Amended and Restated Certificate of Incorporation of SurgiVision, Inc., as amended(1) | |
| 3.2** | Bylaws of SurgiVision, Inc., as amended(1) | |
| 3.3** | Form of Amended and Restated Certificate of Incorporation of SurgiVision, Inc. to be effective upon completion of this offering(5) | |
| 3.4** | Form of Amended and Restated Bylaws of SurgiVision, Inc. to become effective upon completion of this offering(5) | |
| 3.5** | Third Amended and Restated Investor Rights’ Agreement dated September 20, 2006, as amended(1) | |
| 3.6** | First Amended and Restated Stockholders’ Agreement dated April 30, 2004(1) | |
| 3.7** | Certificate of Designation, Preferences and Rights of Series A Convertible Preferred Stock of Surgi-Vision, Inc. filed with the State of Delaware on September 20, 2006(3) | |
| 4.1 | Reference is made to Exhibits 3.1, 3.2, 3.3 and 3.4 | |
| 4.2** | Specimen of Common Stock Certificate(5) | |
| 4.3** | Form of SurgiVision, Inc. 10% Senior Unsecured Convertible Note Due 2012(5) | |
| 4.4** | SurgiVision, Inc. Warrant to Purchase Common Stock, dated March 30, 2010, issued to Gilford Securities Incorporated(5) | |
| 5.1 | Opinion of Baker, Donelson, Bearman, Caldwell & Berkowitz, PC | |
| 10.1** | Surgi-Vision, Inc. 1998 Stock Option Plan(1) | |
| 10.2** | Surgi-Vision, Inc. 2007 Stock Incentive Plan(1) | |
| 10.3** | SurgiVision, Inc. Amended and Restated Key Personnel Incentive Program(7) | |
| 10.4** | 2010 Incentive Compensation Plan(5) | |
| 10.5** | 2010 Incentive Compensation Plan Form of Incentive Stock Option Agreement(7) | |
| 10.6** | 2010 Incentive Compensation Plan Form of Non-Qualified Stock Option Agreement(7) | |
| 10.7** | 2010 Incentive Compensation Plan Form of Non-Qualified Stock Option Agreement for Non-Employee Directors(7) | |
| 10.8** | Form of Indemnification Agreement(5) | |
| 10.9**† | License Agreement by and between SurgiVision, Inc. and The Johns Hopkins University entered into on or around June 20, 1998, as amended by that certain Amendment to License Agreement dated as of January 15, 2000, and as further amended by that certain Addendum to License Agreement entered into on or around December 7, 2004(7) | |
| 10.10**† | License Agreement by and between SurgiVision, Inc. and The Johns Hopkins University entered into on or around December 7, 2006(7) | |
| 10.11**† | Technology License Agreement dated as of December 30, 2005 by and between SurgiVision, Inc. and Boston Scientific Neuromodulation Corporation (formerly known as Advanced Bionics Corporation), as amended by that certain Omnibus Amendment dated June 30, 2007, and as further amended by that certain Omnibus Amendment #2 dated March 19, 2008(7) | |
| 10.12**† | System and Lead Development and Transfer Agreement dated as of December 30, 2005 by and between SurgiVision, Inc. and Boston Scientific Neuromodulation Corporation (formerly known as Advanced Bionics Corporation), as amended by that certain Amendment No. 1 dated May 31, 2006, as further amended by that certain Omnibus Amendment dated June 30, 2007, and as further amended by that certain Omnibus Amendment #2 dated March 19, 2008(7) | |
| Number |
Description | |
| 10.13**† | Technology License Agreement dated as of March 19, 2008 by and between SurgiVision, Inc. and Cardiac Pacemakers, Inc.(6) | |
| 10.14**† | Development Agreement dated as of March 19, 2008 by and between SurgiVision, Inc. and Cardiac Pacemakers, Inc.(6) | |
| 10.15**† | Cooperation and Development Agreement, dated as of May 4, 2009, by and between SurgiVision, Inc. and Siemens Aktiengesellschaft, Healthcare Sector(6) | |
| 10.16** | Consulting Agreement, effective as of May 1, 2009, by and between SurgiVision, Inc. and Dr. Paul Bottomley(4) | |
| 10.17** | Stock Purchase Agreement, dated December 22, 2009, by and between SurgiVision, Inc. and Kimble L. Jenkins(3) | |
| 10.18** | Non-Qualified Stock Option Agreement, dated December 22, 2009, by and between SurgiVision, Inc. and Kimble L. Jenkins(3) | |
| 10.19**† | Patent License Agreement – Nonexclusive entered into on or around April 27, 2009 by and between SurgiVision, Inc. and National Institutes of Health(6) | |
| 10.20**† | Master Services and Licensing Agreement dated as of July 20, 2007 by and between SurgiVision, Inc. and Cedara Software Corp. (6) | |
| 10.21**† | Exclusive License Agreement entered into on or around June 30, 2008 by and between SurgiVision, Inc. and The Johns Hopkins University(6) | |
| 10.22**† | Exclusive License Agreement entered into on or around June 30, 2008 by and between SurgiVision, Inc. and The Johns Hopkins University(6) | |
| 10.23**† | Exclusive License Agreement entered into on or around June 30, 2008 by and between SurgiVision, Inc. and The Johns Hopkins University(6) | |
| 10.24**† | Loan Agreement dated as of October 16, 2009 by and between SurgiVision, Inc. and Boston Scientific Corporation(2) | |
| 10.25**† | Patent Security Agreement dated as of October 16, 2009 by and between SurgiVision, Inc. and Boston Scientific Corporation(2) | |
| 10.26**† | Sponsored Research Agreement by and between SurgiVision, Inc. and the Regents of the University of California on behalf of its San Francisco campus entered into on or around August 24, 2007, as amended by that certain First Amendment to Sponsored Research Agreement dated December 1, 2008, as further amended by that certain Second Amendment to Sponsored Research Agreement dated May 1, 2009, as further amended by that certain Third Amendment to Sponsored Research Agreement dated November 2, 2009, as further amended by that certain Addendum to Sponsored Research Agreement dated February 4, 2010(6) | |
| 10.27**† | Research Agreement by and between SurgiVision, Inc. and The University of Utah entered into on or around July 2, 2007, as amended by that certain First Amendment to the Research Agreement entered into on or around January 8, 2008, as further amended by that certain Second Amendment to the Research Agreement dated April 24, 2009, as further amended by that certain Third Amendment to the Research Agreement dated May 1, 2009, as further amended by that certain Fourth Amendment to the Research Agreement entered into on or around February 25, 2010(6) | |
| 10.28** | Lease Agreement, dated as of April 21, 2008, by and between Shaw Investment Company, LLC and Surgi-Vision, Inc.(5) | |
| 10.29** | Separation Agreement, dated as of April 30, 2010, by and between John Thomas and SurgiVision, Inc.(7) | |
| 10.30** |
SurgiVision, Inc. Cardiac EP Business Participation Plan(7) | |
| Number |
Description | |
| 10.31** | Cardiac EP Business Participation Plan Award Agreement, dated June 3, 2010, by and between SurgiVision, Inc. and Nassir F. Marrouche(7) | |
| 10.32** | Employment Agreement, dated as of June 3, 2010, by and between SurgiVision, Inc. and Kimble L. Jenkins (8) | |
| 10.33** | Employment Agreement, dated as of June 3, 2010, by and between SurgiVision, Inc. and Peter G. Piferi (8) | |
| 10.34** | Employment Agreement, dated as of June 3, 2010, by and between SurgiVision, Inc. and David W. Carlson (8) | |
| 10.35** | Employment Agreement, dated as of June 3, 2010, by and between SurgiVision, Inc. and Oscar L. Thomas (8) | |
| 10.36** | Employment Agreement, dated as of June 3, 2010, by and between SurgiVision, Inc. and John T. Keane (8) | |
| 10.37** | Employment Agreement, dated as of June 3, 2010, by and between SurgiVision, Inc. and Michael M. Moore (8) | |
| 10.38** | Employment Agreement, dated as of June 3, 2010, by and between SurgiVision, Inc. and Carol J. Barbre (8) | |
| 10.39** | Amended and Restated Key Personnel Incentive Award Agreement, dated June 2, 2010, by and between SurgiVision, Inc. and Paul A. Bottomley(7) | |
| 10.40** | Amended and Restated Key Personnel Incentive Award Agreement, dated June 2, 2010, by and between SurgiVision, Inc. and Paul A. Bottomley(7) | |
| 10.41** | Amended and Restated Key Personnel Incentive Award Agreement, dated June 2, 2010, by and between SurgiVision, Inc. and Parag V. Karmarkar(7) | |
| 10.42** | Form of Non-Competition Agreement(7) | |
| 10.43** | Stock Purchase and Loan Agreement, dated January 30, 2009, by and between SurgiVision, Inc. and DARA Biosciences, Inc.(8) | |
| 10.44** | Secured Promissory Note, dated January 30, 2009, issued by DARA Pharmaceuticals, Inc. to SurgiVision, Inc.(8) | |
| 10.45** | Stock Pledge Agreement, dated January 30, 2009, by and between SurgiVision, Inc. and DARA Pharmaceuticals, Inc.(8) | |
| 10.46** | Stockholder Agreement, dated January 30, 2009, by and between SurgiVision, Inc. and DARA Pharmaceuticals, Inc.(8) | |
| 10.47** | Stock Purchase Agreement, dated December 31, 2009, by and between SurgiVision, Inc. and DARA Pharmaceuticals, Inc.(8) | |
| 21** | List of Subsidiary(9) | |
| 23.1 | Consent of Cherry, Bekaert & Holland, L.L.P. | |
| 23.2** | Consent of Baker, Donelson, Bearman, Caldwell & Berkowitz, PC (included in Exhibit 5.1) | |
| 24.1** | Power of attorney. Reference is made to the signature page.(1) | |
| 24.2** | Power of Attorney(7) | |
| * | To be filed by amendment. |
| ** | Previously filed. |
| † | Confidential treatment requested under 17 C.F.R. §§200.80(b)(4) and 230.406. The confidential portions of this exhibit have been omitted and are marked accordingly. The confidential portions have been filed separately with the Securities and Exchange Commission pursuant to the confidential treatment request. |
| (1) | Previously filed with the Securities and Exchange Commission on December 23, 2009 on the Registrant’s Registration Statement on Form S-1 (SEC File No. 333-163957). |
| (2) | Previously filed with the Securities and Exchange Commission on February 9, 2010 on the Registrant’s Registration Statement on Form S-1/A (SEC File No. 333-163957). |
| (3) | Previously filed with the Securities and Exchange Commission on February 26, 2010 on the Registrant’s Registration Statement on Form S-1/A (SEC File No. 333-163957). |
| (4) | Previously filed with the Securities and Exchange Commission on April 6, 2010 on the Registrant’s Registration Statement on Form S-1/A (SEC File No. 333-163957). |
| (5) | Previously filed with the Securities and Exchange Commission on May 7, 2010 on the Registrant’s Registration Statement on Form S-1/A (SEC File No. 333-163957). |
| (6) | Previously filed with the Securities and Exchange Commission on May 13, 2010 on the Registrant’s Registration Statement on Form S-1/A (SEC File No. 333-163957). |
| (7) | Previously filed with the Securities and Exchange Commission on June 4, 2010 on the Registrant’s Registration Statement on Form S-1/A (SEC File No. 333-163957). |
| (8) | Previously filed with the Securities and Exchange Commission on June 25, 2010 on the Registrant’s Registration Statement on Form S-1/A (SEC File No. 333-163957). |
| (9) | Previously filed with the Securities and Exchange Commission on July 2, 2010 on the Registrant’s Registration Statement on Form S-1/A (SEC File No. 333-163957). |