
TM

© 2 0 2 2 C L E A R P O I N T N E U R O 2 Statements herein concerning the Company’s plans, growth and strategies may include forward-looking statements within the context of the federal securities laws. Statements regarding the Company's future events, developments and future performance, as well as management's expectations, beliefs, plans, estimates or projections relating to the future, are forward-looking statements within the meaning of these laws. Uncertainties and risks may cause the Company's actual results to differ materially from those expressed in or implied by forward-looking statements. Particular uncertainties and risks include those relating to: the impact of the COVID-19 pandemic and the measures adopted to contain its spread; future revenue from sales of the Company’s ClearPoint Neuro Navigation System products; the Company’s ability to market, commercialize and achieve broader market acceptance for the Company’s ClearPoint Neuro Navigation System products; the ability of our biologics and drug delivery partners to achieve commercial success, including their use of our products and services in their delivery of therapies; and risks inherent in the research and development of new products. More detailed information on these and additional factors that could affect the Company’s actual results are described in the “Risk Factors” section of the Company’s Annual Report on Form 10-K for the year ended December 31, 2021, and the Company’s Quarterly Report on Form 10-Q for the three months ended March 31, 2022, both of which have been filed with the Securities and Exchange Commission, and the Company’s Quarterly Report on Form 10-Q for the three months ended June 30, 2022, which the Company intends to file with the Securities and Exchange Commission on or before August 15, 2022.
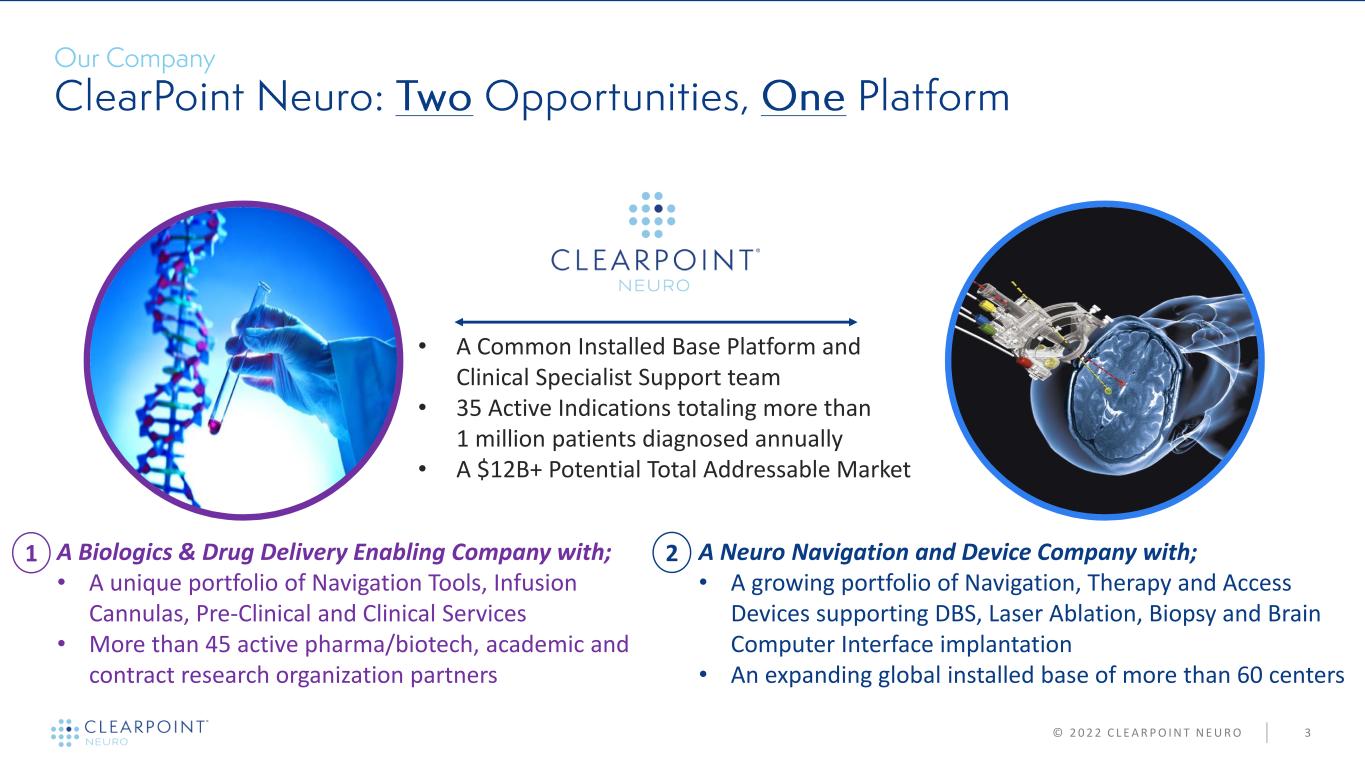
© 2 0 2 2 C L E A R P O I N T N E U R O 3 A Neuro Navigation and Device Company with; • A growing portfolio of Navigation, Therapy and Access Devices supporting DBS, Laser Ablation, Biopsy and Brain Computer Interface implantation • An expanding global installed base of more than 60 centers A Biologics & Drug Delivery Enabling Company with; • A unique portfolio of Navigation Tools, Infusion Cannulas, Pre-Clinical and Clinical Services • More than 45 active pharma/biotech, academic and contract research organization partners • A Common Installed Base Platform and Clinical Specialist Support team • 35 Active Indications totaling more than 1 million patients diagnosed annually • A $12B+ Potential Total Addressable Market 1 2
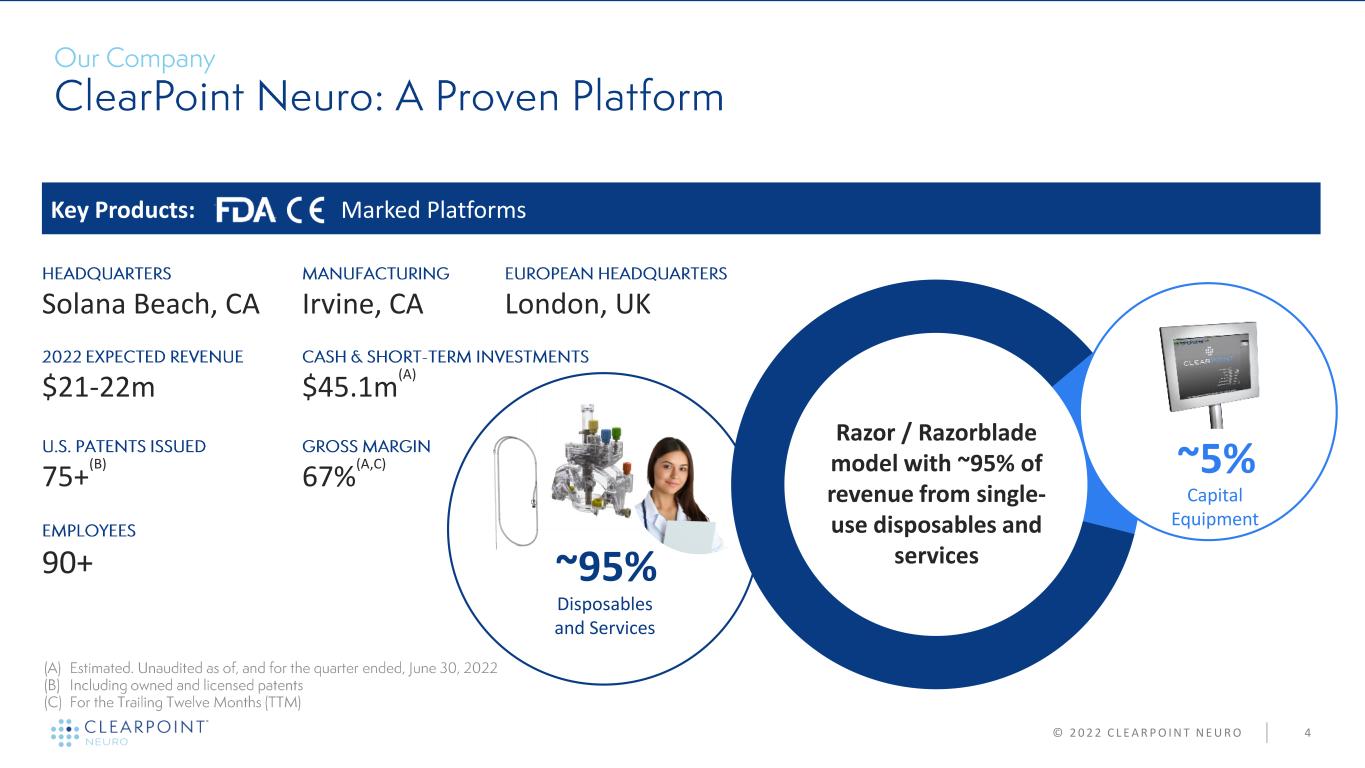
4 90+ $21-22m $45.1m (A) Solana Beach, CA Key Products: Marked Platforms Capital Equipment Razor / Razorblade model with ~95% of revenue from single- use disposables and services 67% (A,C) ~5% ~95% Disposables and Services © 2 0 2 2 C L E A R P O I N T N E U R O Irvine, CA London, UK 75+ (B)
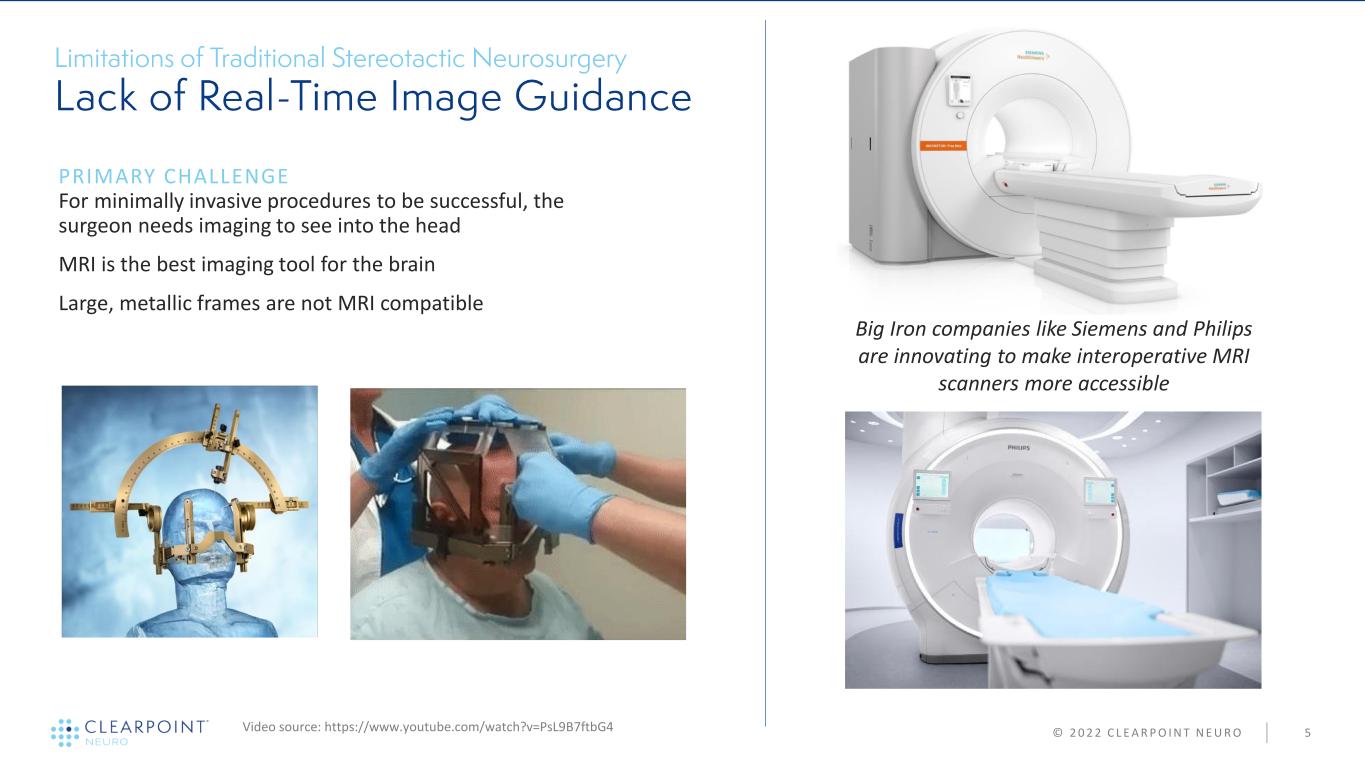
© 2 0 2 2 C L E A R P O I N T N E U R O 5 PRIMARY CHALLENGE For minimally invasive procedures to be successful, the surgeon needs imaging to see into the head MRI is the best imaging tool for the brain Large, metallic frames are not MRI compatible Video source: https://www.youtube.com/watch?v=PsL9B7ftbG4 Big Iron companies like Siemens and Philips are innovating to make interoperative MRI scanners more accessible
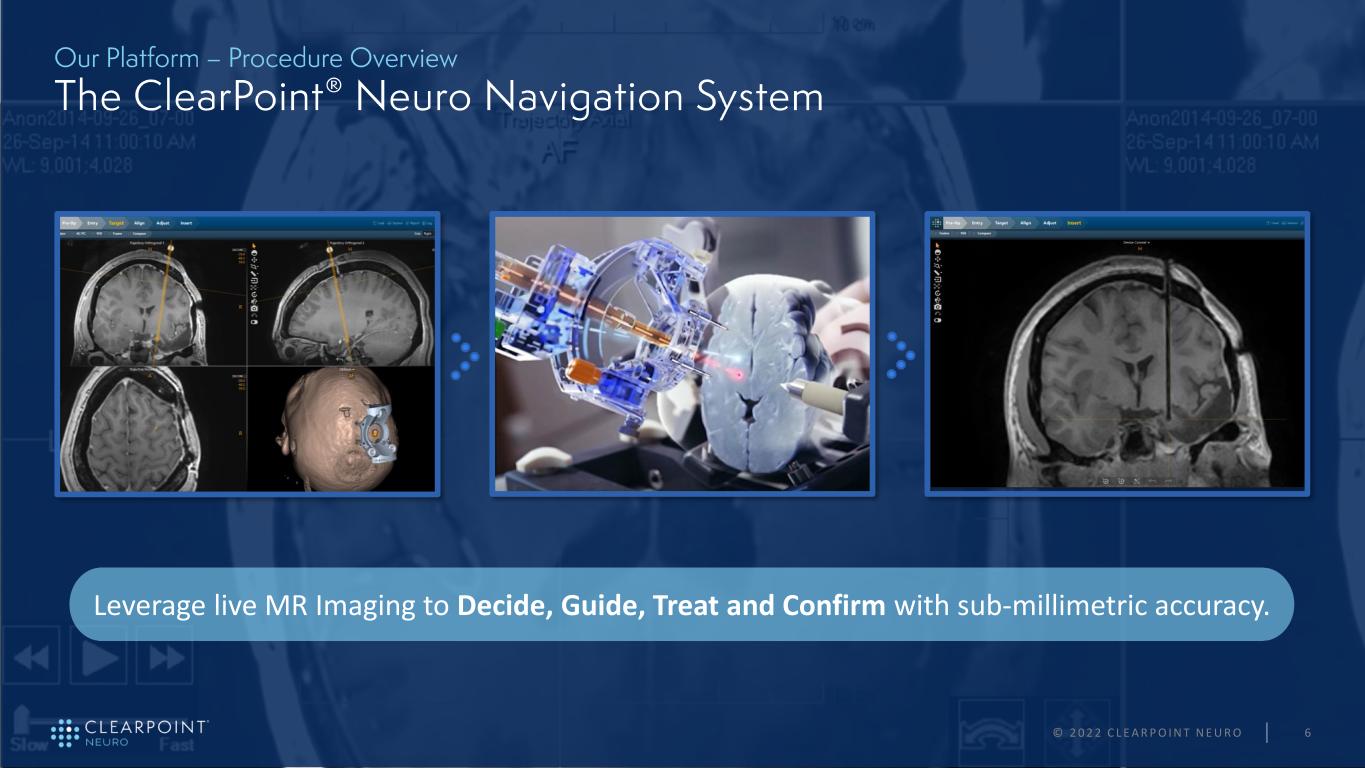
© 2 0 2 2 C L E A R P O I N T N E U R O 6 Leverage live MR Imaging to Decide, Guide, Treat and Confirm with sub-millimetric accuracy.
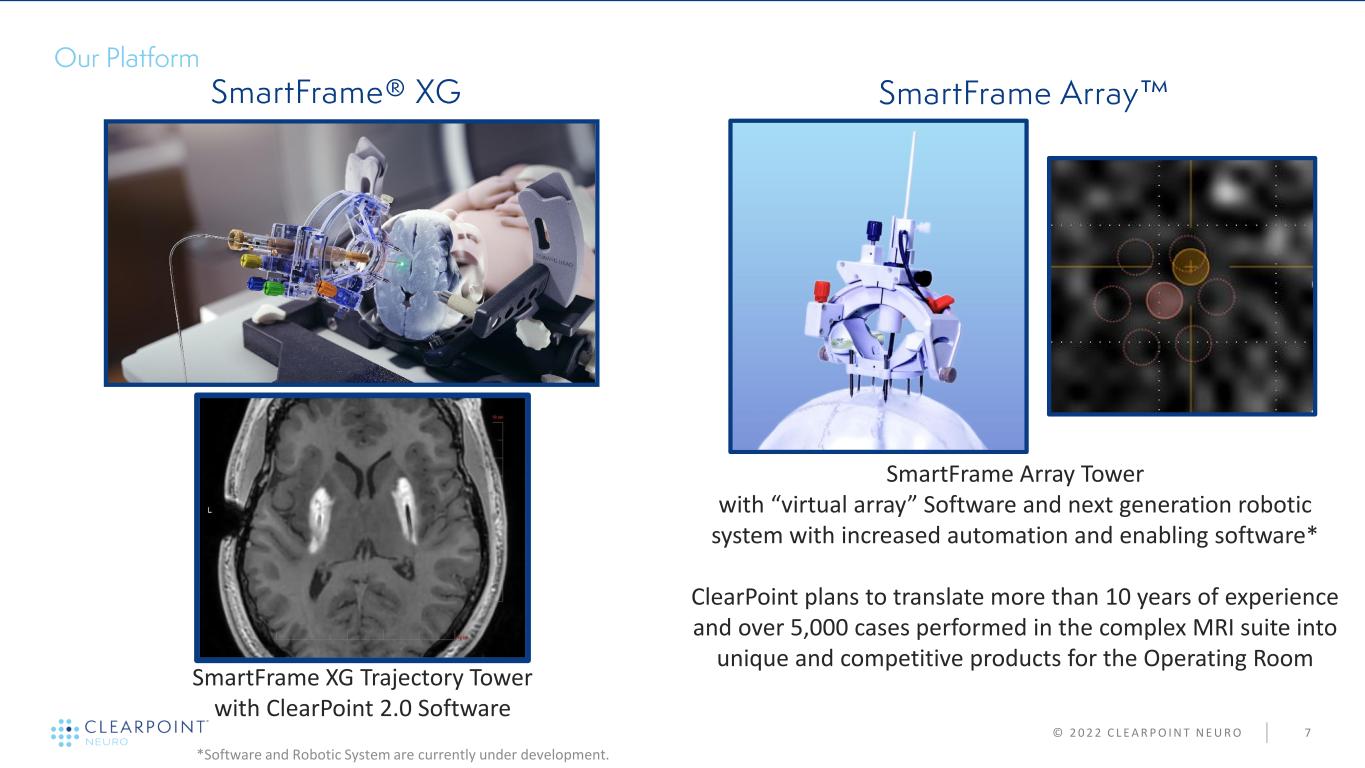
© 2 0 2 2 C L E A R P O I N T N E U R O 7 SmartFrame XG Trajectory Tower with ClearPoint 2.0 Software SmartFrame Array Tower with “virtual array” Software and next generation robotic system with increased automation and enabling software* ClearPoint plans to translate more than 10 years of experience and over 5,000 cases performed in the complex MRI suite into unique and competitive products for the Operating Room *Software and Robotic System are currently under development.
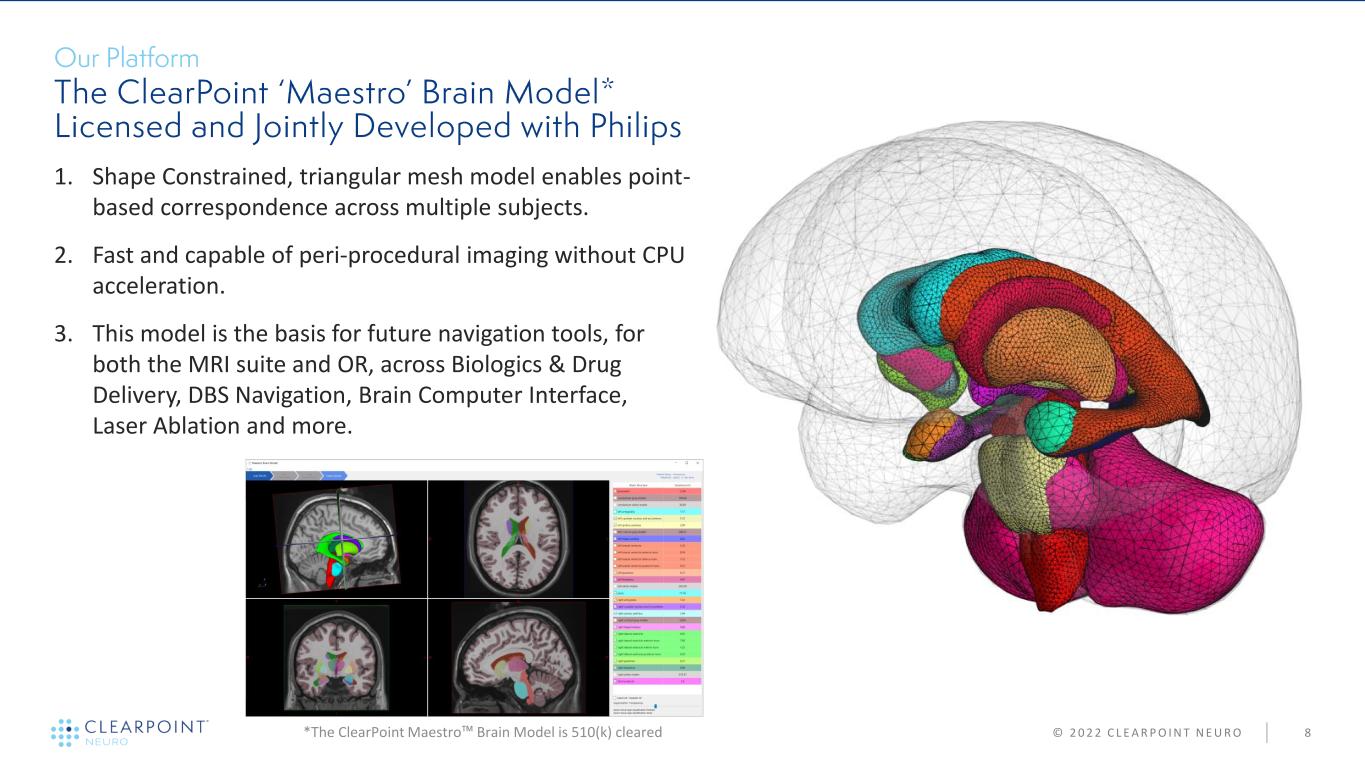
© 2 0 2 2 C L E A R P O I N T N E U R O 8 1. Shape Constrained, triangular mesh model enables point- based correspondence across multiple subjects. 2. Fast and capable of peri-procedural imaging without CPU acceleration. 3. This model is the basis for future navigation tools, for both the MRI suite and OR, across Biologics & Drug Delivery, DBS Navigation, Brain Computer Interface, Laser Ablation and more. *The ClearPoint Maestro™ Brain Model is 510(k) cleared

© 2 0 2 1 C L E A R P O I N T N E U R O 9

10 PLACE LASER ABLATION PROBES VISUALASE CLPT PLATFORM RUNS ON ALL MAJOR SCANNERS © 2 0 2 2 C L E A R P O I N T N E U R O IMPLANT NEURO STIMULATION LEADS … More than 45 active pharma/biotech, academic, and contract research organization partners DELIVER BIOLOGICS AND DRUGS *In partnership with Clinical Laserthermia Systems AB. 510(k) pending
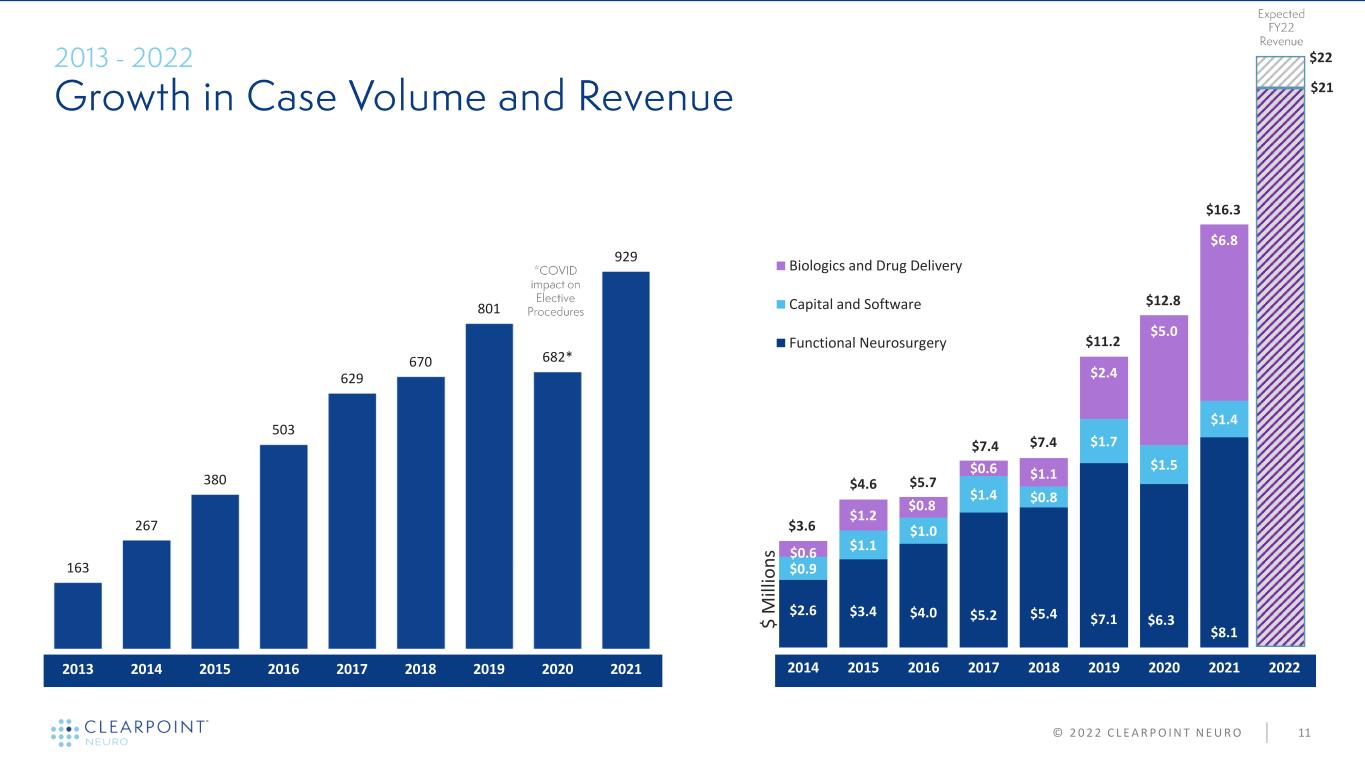
$2.6 $3.4 $4.0 $5.2 $5.4 $7.1 $6.3 $8.1 $0.9 $1.1 $1.0 $1.4 $0.8 $1.7 $1.5 $1.4 $0.6 $1.2 $0.8 $0.6 $1.1 $2.4 $5.0 $6.8 2014 2015 2016 2017 2018 2019 2020 2021 2022 Biologics and Drug Delivery Capital and Software Functional Neurosurgery 163 267 380 503 629 670 801 682* 929 2013 2014 2015 2016 2017 2018 2019 2020 2021 11© 2 0 2 2 C L E A R P O I N T N E U R O $ M ill io n s $7.4 $11.2 $12.8 $7.4 $5.7$4.6 $3.6 $16.3 $21 $22
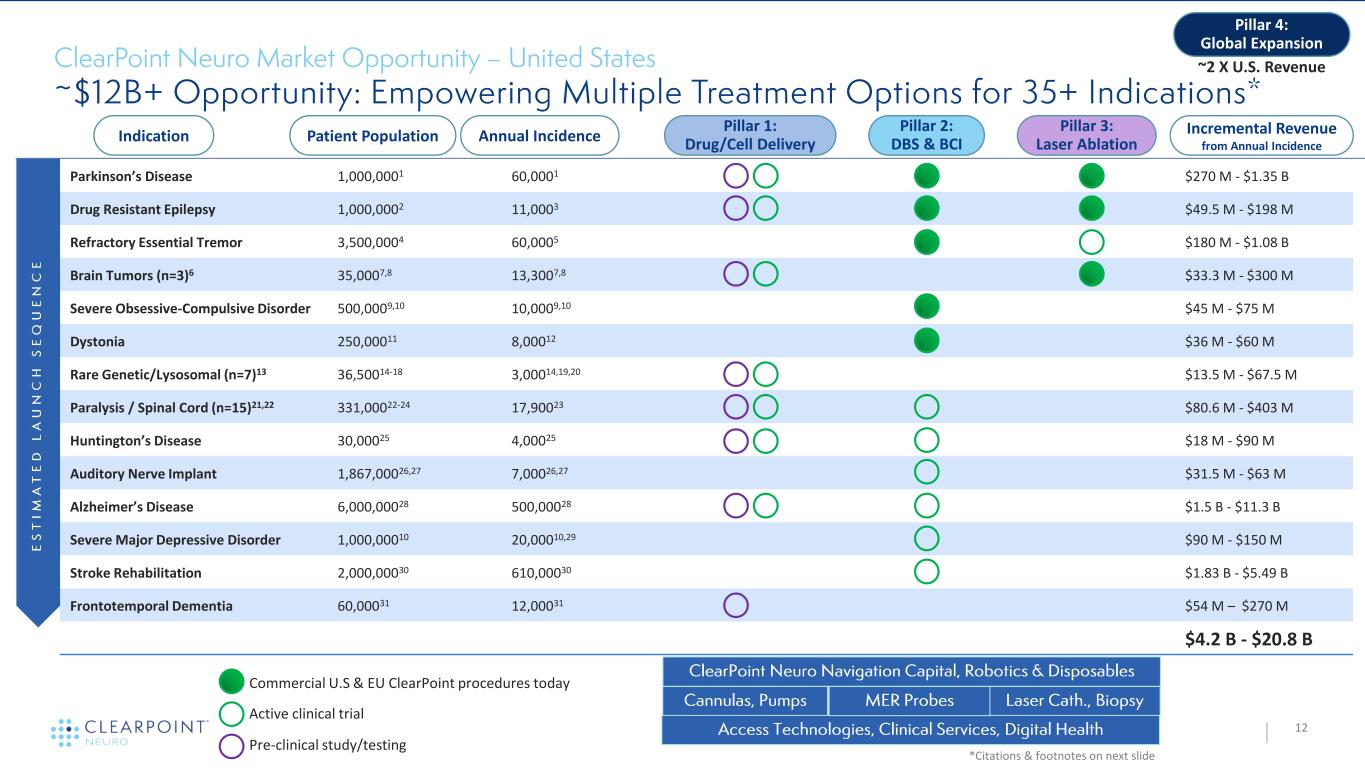
Parkinson’s Disease 1,000,0001 60,0001 • $270 M - $1.35 B Drug Resistant Epilepsy 1,000,0002 11,0003 • $49.5 M - $198 M Refractory Essential Tremor 3,500,0004 60,0005 $180 M - $1.08 B Brain Tumors (n=3)6 35,0007,8 13,3007,8 $33.3 M - $300 M Severe Obsessive-Compulsive Disorder 500,0009,10 10,0009,10 $45 M - $75 M Dystonia 250,00011 8,00012 $36 M - $60 M Rare Genetic/Lysosomal (n=7)13 36,50014-18 3,00014,19,20 $13.5 M - $67.5 M Paralysis / Spinal Cord (n=15)21,22 331,00022-24 17,90023 $80.6 M - $403 M Huntington’s Disease 30,00025 4,00025 $18 M - $90 M Auditory Nerve Implant 1,867,00026,27 7,00026,27 $31.5 M - $63 M Alzheimer’s Disease 6,000,00028 500,00028 $1.5 B - $11.3 B Severe Major Depressive Disorder 1,000,00010 20,00010,29 $90 M - $150 M Stroke Rehabilitation 2,000,00030 610,00030 $1.83 B - $5.49 B Frontotemporal Dementia 60,00031 12,00031 $54 M – $270 M $4.2 B - $20.8 B © 2 0 2 1 C L E A R P O I N T N E U R O 12 *Citations & footnotes on next slide Indication Patient Population Annual Incidence Pillar 2: DBS & BCI Pillar 3: Laser Ablation Pillar 1: Drug/Cell Delivery Incremental Revenue from Annual Incidence Pillar 4: Global Expansion Commercial U.S & EU ClearPoint procedures today ~2 X U.S. Revenue Active clinical trial Pre-clinical study/testing

© 2 0 2 2 C L E A R P O I N T N E U R O 13 1. “Parkinson’s Disease Statistics,” Parkinson’s News Today, https://parkinsonsnewstoday.com/parkinsons-disease-statistics/#:~:text=An%20estimated%20seven%20to%2010,who%20are%2080%20and%20older 2. Neurona Therapeutics. (2021 November 4). Neurona Therapeutics Receives IND Clearance to Initiate Phase 1/2 Clinical Trial of Neural Cell Therapy NRTX-1001 in Chronic Focal Epilepsy Patients [Press release] https://www.neuronatherapeutics.com/wp-content/uploads/2021/11/2021_11_01_-INDClearance_FINALVersion.pdf 3. Asadi-Pooya AA, Stewart GR, Abrams DJ, Sharan A. Prevalence and Incidence of Drug-Resistant Mesial Temporal Lobe Epilepsy in the United States. World Neurosurg. 2017;99:662-666. 4. Zesiewicz TA, Chari A, Jahan I, Miller AM, Sullivan KL. Overview of essential tremor. Neuropsychiatr Dis Treat. 2010;6:401-408. Published 2010 Sep 7. 5. Diaz NL, Louis ED. Survey of medication usage patterns among essential tremor patients: movement disorder specialists vs. general neurologists. Parkinsonism Relat Disord. 2010;16(9):604-607. 6. Includes: Glioblastoma, Diffuse Intrinsic Pontine Glioma and deep small eloquent brain tumors. 7. “Glioblastoma Multiforme,” American Association of Neurological Surgeons, https://www.aans.org/en/Patients/Neurosurgical-Conditions-and-Treatments/Glioblastoma-Multiforme 8. “About DIPG/DMG,” DIPG/DMG Registry, https://dipgregistry.org/patients-families/about-dipg-dmg/ 9. Medtronic Clinical Summary – Reclaim DBS for Chronic Extreme OCD M947128A001. 10. Mantovani A, Lisanby SH. Brain stimulation in the treatment of anxiety disorders. In: Simpson HB, Neria Y, Lewis-Fernández R, Schneier F, eds. Anxiety Disorders: Theory, Research and Clinical Perspectives. Cambridge: Cambridge University Press; 2010:323-335. 11. https://www.aans.org/en/Patients/Neurosurgical-Conditions-and-Treatments/Dystonia 12. Medtronic DBS™ Therapy for Dystonia - Clinical Summary 2015. 13. Includes: AADC deficiency, Friedreich’s ataxia, Angelman syndrome, multiple system atrophy, metachromatic leukodystrophy, and spinocerebellar ataxia type 3. 14. "Multiple System Atrophy," Medscape, https://emedicine.medscape.com/article/1154583-overview#a6 15. PTC Therapeutics November 30, 2021 Corporate Presentation, https://ir.ptcbio.com/static-files/0fd5d54f-55b8-416b-8006-4eb4c0d82f45 16. “Spinocerebellar ataxia type 3,” Orphanet, https://www.orpha.net/consor/cgi-bin/OC_Exp.php?lng=EN&Expert=98757 17. Lysogene Corporate Presentation at 38th Annual J.P. Morgan Healthcare Conference on Jan 13, 2020, http://www.lysogene.com/wp-content/uploads/2020/01/jpm-2020-corporate-presentation_final.pdf 18. “Metachromatic Leukodystrophy,” National Organization of Rare Disorders, https://rarediseases.org/rare-diseases/metachromatic-leukodystrophy/ 19. “Aromatic L’Amino Acid Decarboxylase Deficiency,” National Organization for Rare Disorders, https://rarediseases.org/rare-diseases/aromatic-l-amino-acid-decarboxylase-deficiency/ 20. Puckett Y, Mallorga-Hernández A, Montaño AM. Epidemiology of mucopolysaccharidoses (MPS) in United States: challenges and opportunities. Orphanet J Rare Dis. 2021;16(1):241. Published 2021 May 29. 21. Includes: stroke, spinal cord injury, multiple sclerosis, cerebral palsy, other (traumatic brain injury, complications from surgery, amyotrophic lateral sclerosis, neurofibromatosis, Chiari malformation, syringomyelia, postpolio syndrome, spinal muscular atrophy, Friedreich’s ataxia, transverse myelitis, and spina bifida). 22. Armour BS, Courtney-Long EA, Fox MH, Fredine H, Cahill A. Prevalence and Causes of Paralysis-United States, 2013. Am J Public Health. 2016;106(10):1855-1857. 23. Wyndaele M, Wyndaele JJ. Incidence, prevalence and epidemiology of spinal cord injury: what learns a worldwide literature survey?. Spinal Cord. 2006;44(9):523-529. 24. National Spinal Cord Injury Statistical Center (NSCISC): 2020 Annual Report and 2021 Facts and Figures. https://www.nscisc.uab.edu/ 25. “Huntington’s Disease,” Mov Disord. 2019 Jun; 34(6): 858–865. 26. “Cochlear Implants,” National Institute on Deafness and Other Communication Disorders, https://www.nidcd.nih.gov/health/cochlear-implants 27. “Cochlear Implant Surgery,” Medscape, https://emedicine.medscape.com/article/857242-overview 28. “Alzheimer’s Disease: Facts & Figures,” Brightfocus Foundation, https://www.brightfocus.org/alzheimers/article/alzheimers-disease-facts-figures 29. Goodman WK, Alterman RL. Deep brain stimulation for intractable psychiatric disorders. Annu Rev Med. 2012;63:511-524. 30. “Stroke Facts,” Center for Disease Control and Prevention, https://www.cdc.gov/stroke/facts.htm 31. Onyike CU, Diehl-Schmid J. The epidemiology of frontotemporal dementia. Int Rev Psychiatry. 2013;25(2):130-137.
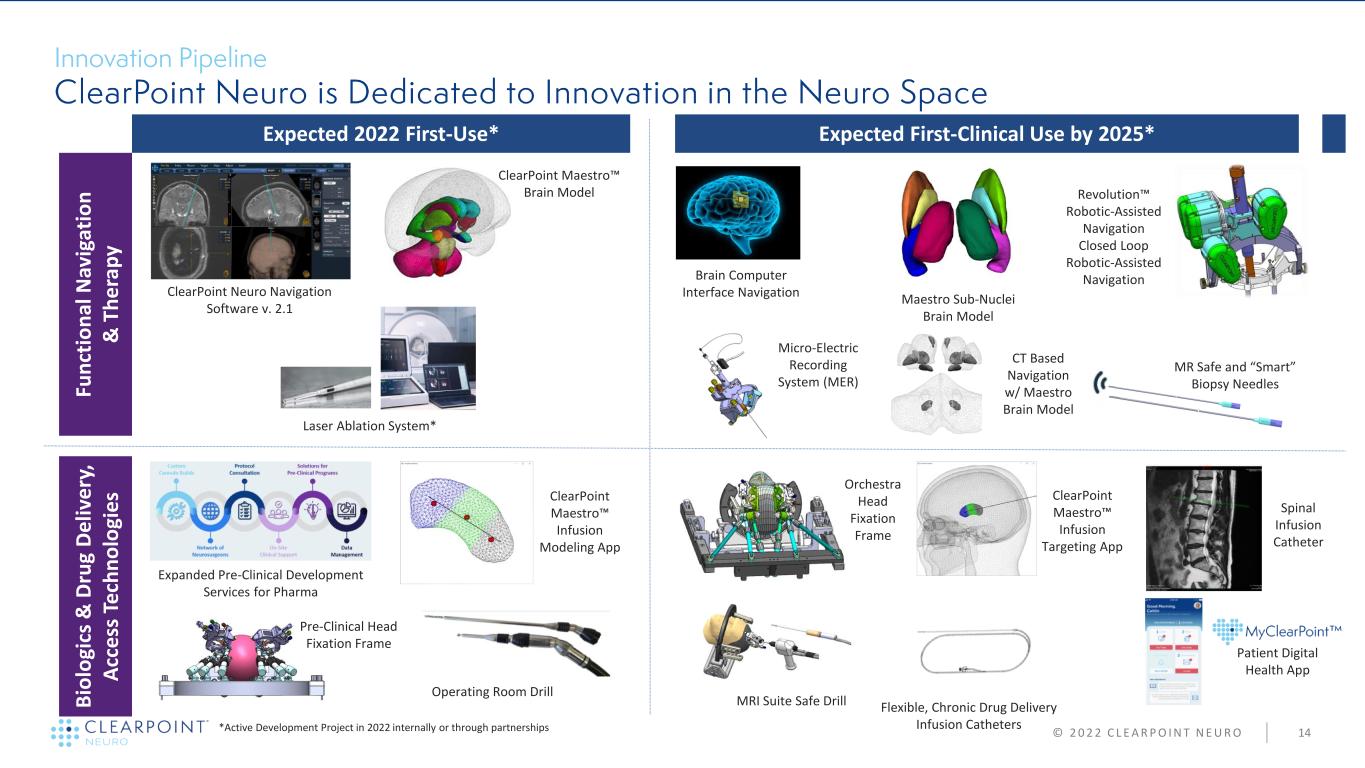
© 2 0 2 2 C L E A R P O I N T N E U R O 14 Expected 2022 First-Use* Expected First-Clinical Use by 2025* Fu n ct io n al N av ig at io n & T h e ra p y B io lo gi cs & D ru g D e liv e ry , A cc e ss T e ch n o lo gi e s ClearPoint Neuro Navigation Software v. 2.1 ClearPoint Maestro™ Brain Model Orchestra Head Fixation Frame Maestro Sub-Nuclei Brain Model Revolution™ Robotic-Assisted Navigation Closed Loop Robotic-Assisted Navigation *Active Development Project in 2022 internally or through partnerships MRI Suite Safe Drill Operating Room Drill Laser Ablation System* MR Safe and “Smart” Biopsy Needles Brain Computer Interface Navigation Spinal Infusion Catheter Pre-Clinical Head Fixation Frame Expanded Pre-Clinical Development Services for Pharma ClearPoint Maestro™ Infusion Modeling App Micro-Electric Recording System (MER) ClearPoint Maestro™ Infusion Targeting App CT Based Navigation w/ Maestro Brain Model Flexible, Chronic Drug Delivery Infusion Catheters Patient Digital Health App

© 2 0 2 2 C L E A R P O I N T N E U R O 15 • Simulated training experience for surgeons and staff to perform mock procedures and provide development pipeline feedback • Showcase new technologies to Pharmaceutical and BCI partners • Home for Clinical Specialists to support cases remotely without travel
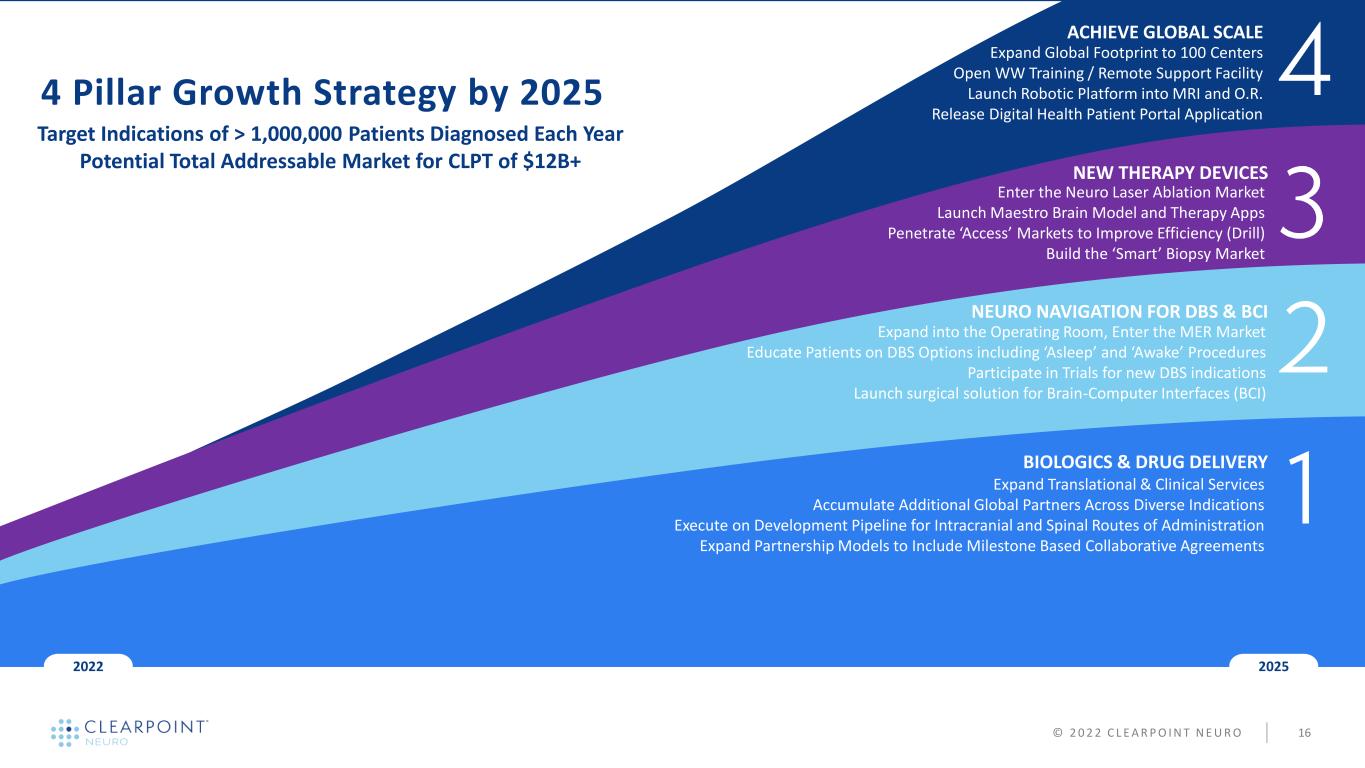
NEW THERAPY DEVICES NEURO NAVIGATION FOR DBS & BCI BIOLOGICS & DRUG DELIVERY 20252022 4 Pillar Growth Strategy by 2025 Target Indications of > 1,000,000 Patients Diagnosed Each Year Potential Total Addressable Market for CLPT of $12B+ Enter the Neuro Laser Ablation Market Launch Maestro Brain Model and Therapy Apps Penetrate ‘Access’ Markets to Improve Efficiency (Drill) Build the ‘Smart’ Biopsy Market ACHIEVE GLOBAL SCALE Expand Global Footprint to 100 Centers Open WW Training / Remote Support Facility Launch Robotic Platform into MRI and O.R. Release Digital Health Patient Portal Application © 2 0 2 2 C L E A R P O I N T N E U R O 16 Expand Translational & Clinical Services Accumulate Additional Global Partners Across Diverse Indications Execute on Development Pipeline for Intracranial and Spinal Routes of Administration Expand Partnership Models to Include Milestone Based Collaborative Agreements Expand into the Operating Room, Enter the MER Market Educate Patients on DBS Options including ‘Asleep’ and ‘Awake’ Procedures Participate in Trials for new DBS indications Launch surgical solution for Brain-Computer Interfaces (BCI)

© 2 0 2 2 C L E A R P O I N T N E U R O 17 Unique platform technology enabling Precision MRI-Guided Therapies to restore quality of life for some of the most debilitating disorders Large, growing installed base in 60+ of 500+ leading Neurosurgery and research centers Worldwide Expandable Platform with realizable synergies beyond the MRI and into the operating room and radiology suite Pipeline of new revenue streams from product improvements, biologic and drug delivery partnerships and services, standalone therapy products, and Brain Computer Interface Navigation Total potential addressable market > $12B for our products, pipeline and partnerships A growing and passionate team of embedded scientists and specialists

© 2 0 2 2 C L E A R P O I N T N E U R O
