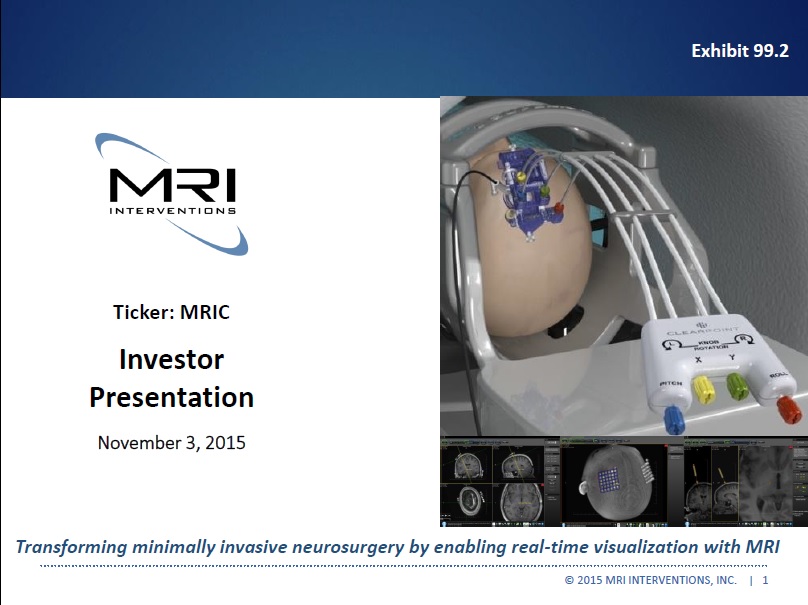
Exhibit 99.2
Ticker: MRIC
Investor Presentation
November 3, 2015
Transforming minimally invasive neurosurgery by enabling real-time visualization with MRI
© 2015 MRI INTERVENTIONS, INC. | 1
 |
Exhibit 99.2
Ticker: MRIC
Investor Presentation
November 3, 2015
Transforming minimally invasive neurosurgery by enabling real-time visualization with MRI
© 2015 MRI INTERVENTIONS, INC. | 1
|
 |
Forward Looking Statements
Certain statements in this presentation may constitute forward-looking statements within the meaning of Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934. Forward-looking statements often can be identified by words such as "anticipates," "believes," "could," "estimates," "expects," "intends," "may," "plans," "potential," "predicts," "projects," "should," "will," "would," or the negative of these words or other words of similar meaning. Forward-looking statements by their nature address matters that, to different degrees, are uncertain and involve risk. Uncertainties and risks may cause MRI Interventions' actual results and the timing of events to differ materially from those expressed in or implied by MRI Interventions' forward-looking statements. Particular uncertainties and risks include, among others: demand and market acceptance of our products; our ability to successfully expand, and achieve full productivity from, our sales, clinical support and marketing capabilities; availability and adequacy of reimbursement from third party payors for procedures utilizing our products; the sufficiency of our cash resources to maintain planned commercialization efforts and research and development programs; future actions of the FDA or any other regulatory body that could impact product development, manufacturing or sale; our ability to protect and enforce our intellectual property rights; our dependence on collaboration partners; the impact of competitive products and pricing; the impact of the commercial and credit environment on us and our customers and suppliers; and our ability to successfully complete the development of, and to obtain regulatory clearance or approval for, our ClearTrace system. More detailed information on these and additional factors that could affect MRI Interventions' actual results and the timing of events are described in its filings with the Securities and Exchange Commission. Except as required by law, MRI Interventions undertakes no obligation to publicly update or revise any forward-looking statements made in this presentation to reflect any change in MRI Interventions' expectations or any change in events, conditions or circumstances on which any such statements are based.
© 2015 MRI INTERVENTIONS, INC. | 2
|
 |
MRI Interventions Opportunity
Market
Market is large and growing
55,000 potential ClearPoint procedures across multiple therapies
Navigation System for Multiple Therapies
Electrode placement for Deep Brain Stimulation
Laser Ablation for ablation of epileptic foci or Brain Tumors
Brain Tumor Biopsy for deep seated tumors
Precise Drug Delivery to target lesions
Large Opportunity Attracting Multiple Players
Area of interest to large medical device companies
Medtronic, St. Jude and Boston Scientific investing in neuro market
MRI Scanner Companies embracing MRI-guided therapies
Drug Companies pursuing direct delivery
Uniquely Positioned
Focused commercial effort; FDA/CE approved products
Delivery platform for multiple therapies
Strong, proprietary position
Recent restructuring complete
© 2015 MRI INTERVENTIONS, INC. | 3
|
 |
MRI Interventions: Real Time MRI Guided Surgery First-to-market technology platform enabling real-time MRI guided surgery; FDA-cleared, CE-marked and 40+ ClearPoint sites Focused commercialization of neuro platform underway, gaining traction; recent restructuring complete Attractive razor/razorblade business model with strong margins Compatible with all major MRI manufacturers; Interoperability w/ Medtronic, Monteris, neuro products Strong intellectual property portfolio Strong management team with extensive medical device commercialization experience: Intuitive, Medtronic, Kyphon, Boston Scientific, Edwards Lifescience, Cordis © 2015 MRI INTERVENTIONS, INC. | 4 |
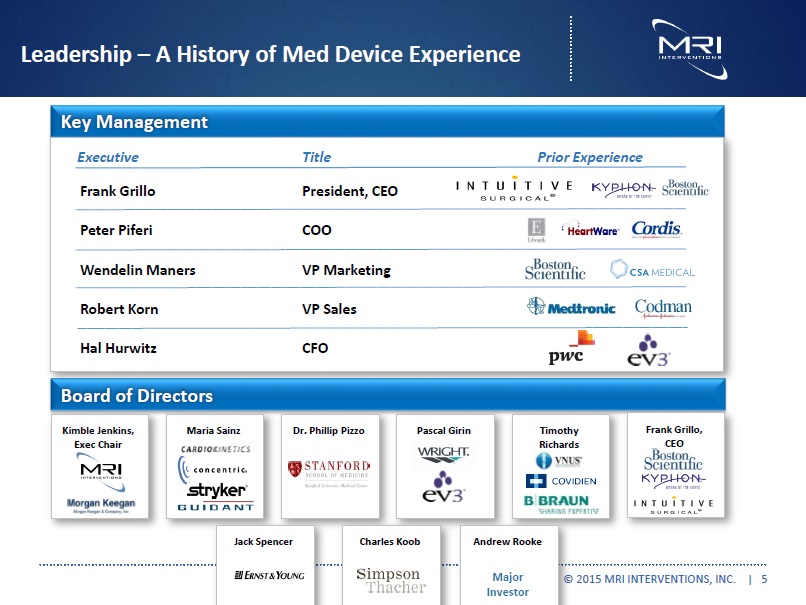 |
Leadership –Significant Med Device Experience Key Management Executive Title Prior Experience Frank Grillo President, CEO Peter Piferi COO Wendelin Maners VP Marketing Robert Korn VP Sales Hal Hurwitz CFO Board of Directors Kimble Jenkins, Exec Chair Maria Sainz Dr. Phillip Pizzo Pascal Girin Timothy Richards Frank Grillo, CEO Jack Spencer Charles Koob Andrew Rooke Major Investor © 2015 MRI INTERVENTIONS, INC. | 5 |
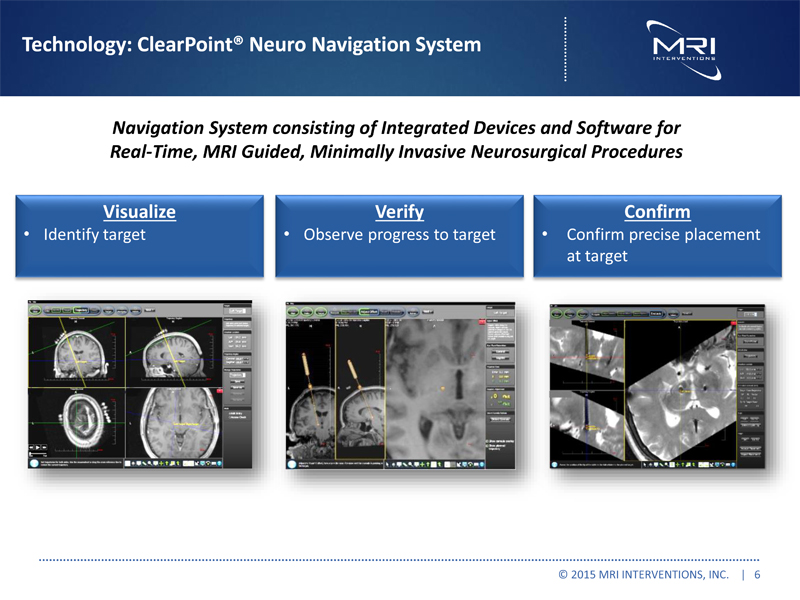 |
Technology: ClearPoint® Neuro Navigation System Navigation System consisting of Integrated Devices and Software for Real-Time, MRI Guided, Minimally Invasive Neurosurgical Procedures Visualize •Identify target Verify •Observe progress to target Confirm •Confirm precise placement at target © 2015 MRI INTERVENTIONS, INC. | 6 |
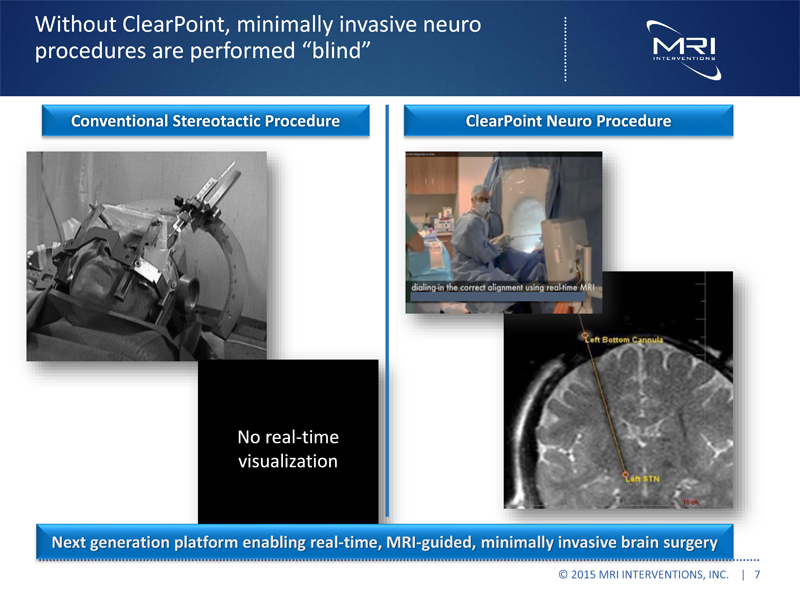 |
Without ClearPoint, minimally invasive neuro procedures are performed “blind” Conventional Stereotactic Procedure ClearPoint Neuro Procedure No real-time visualization Next generation platform enabling real time, MRI guided, minimally invasive brain surgery © 2015 MRI INTERVENTIONS, INC. | 7 |
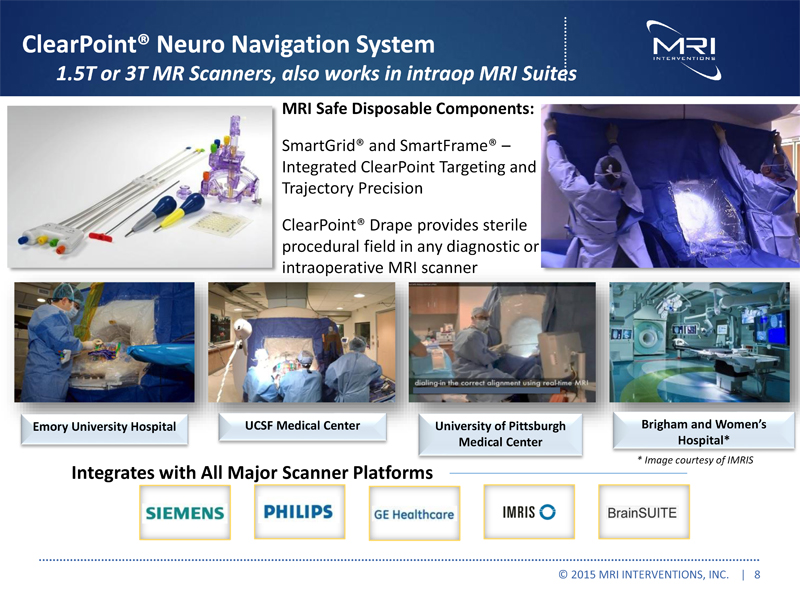 |
ClearPoint® Neuro Navigation System 1.5T or 3T MR Scanners, also works in intraop MRI Suites MRI Safe Disposable Components: SmartGrid® and SmartFrame® – Integrated ClearPoint Targeting and Trajectory Precision ClearPoint® Drape provides sterile procedural field in any diagnostic or intraoperative MRI scanner Emory University Hospital UCSF Medical Center University of Pittsburgh Medical Center Brigham and Women’s Hospital* * Image courtesy of IMRIS Integrates with All Major Scanner Platforms © 2015 MRI INTERVENTIONS, INC. | 8 |
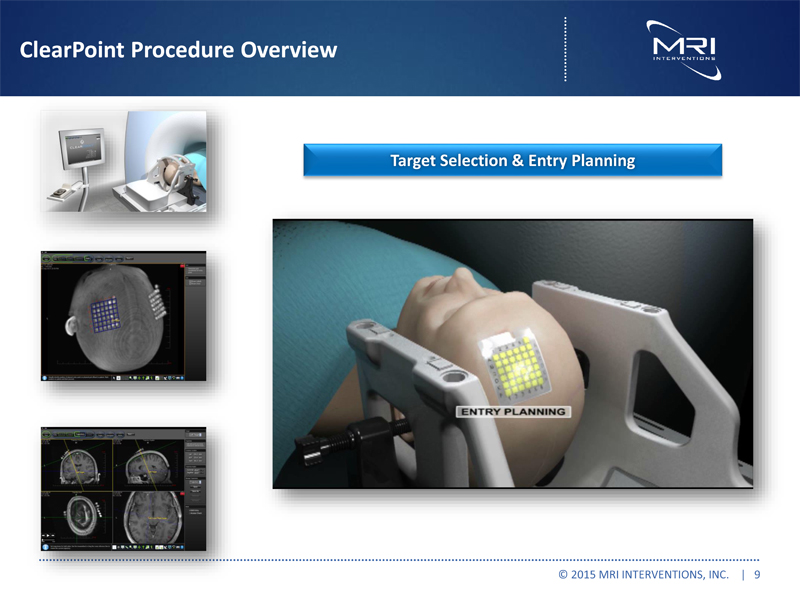 |
ClearPoint Procedure Overview Target Selection & Entry Planning © 2015 MRI INTERVENTIONS, INC. | 9 |
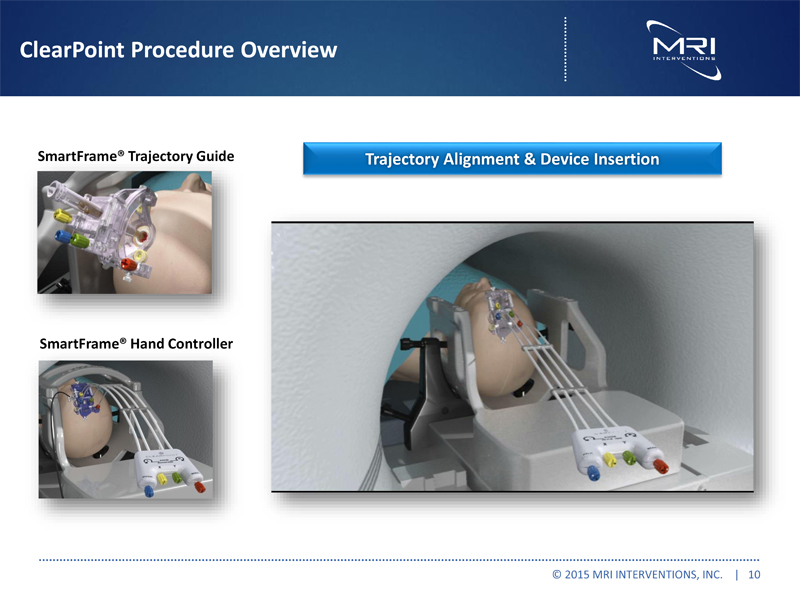 |
ClearPoint Procedure Overview SmartFrame® Trajectory Guide SmartFrame® Hand Controller Trajectory Alignment & Device Insertion © 2015 MRI INTERVENTIONS, INC. | 10 |
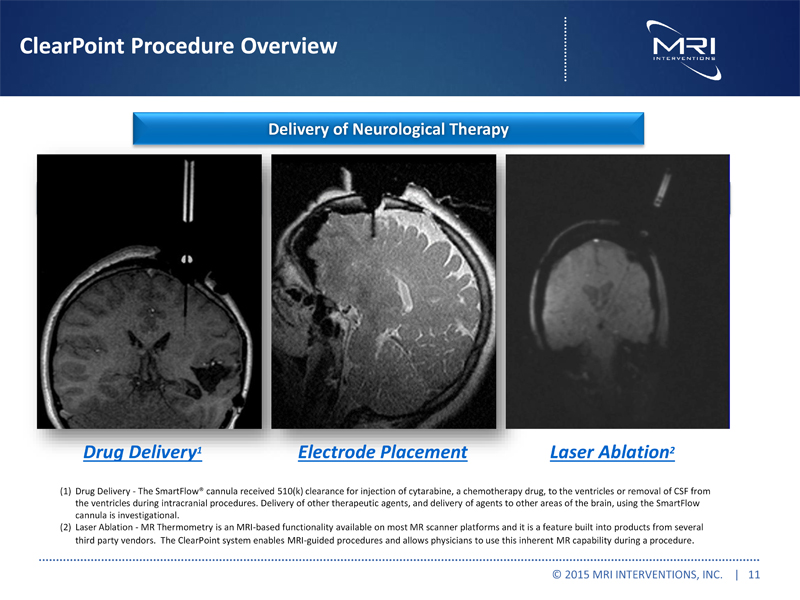 |
ClearPoint Procedure Overview Delivery of Neurological Therapy Drug Delivery1 Electrode Placement Laser Ablation2 (1) Drug Delivery - The SmartFlow® cannula received 510(k) clearance for injection of cytarabine, a chemotherapy drug, to the ventricles or removal of CSF from the ventricles during intracranial procedures. Delivery of other therapeutic agents, and delivery of agents to other areas of the brain, using the SmartFlow cannula is investigational. (2) Laser Ablation - MR Thermometry is an MRI-based functionality available on most MR scanner platforms and it is a feature built into products from several third party vendors. The ClearPoint system enables MRI-guided procedures and allows physicians to use this inherent MR capability during a procedure. © 2015 MRI INTERVENTIONS, INC. | 11 |
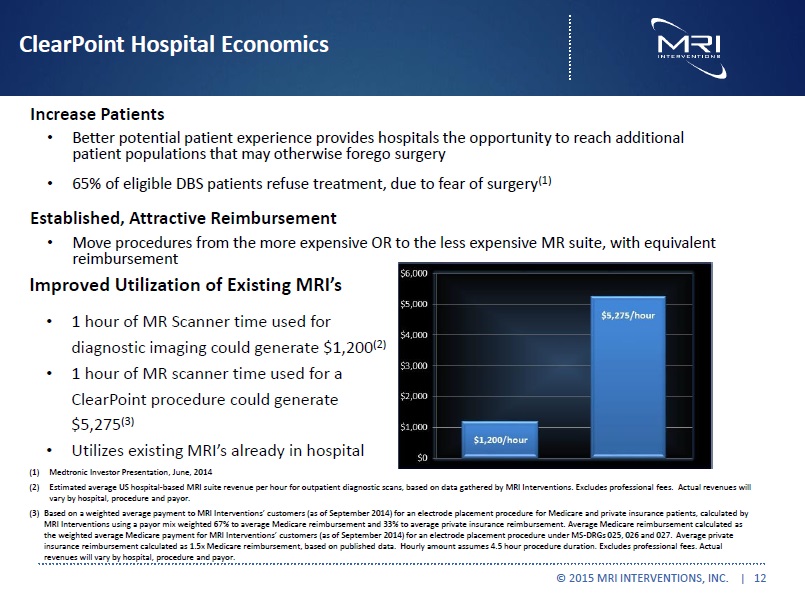 |
ClearPoint Hospital Economics Increase Patients Better patient experience provides hospitals the opportunity to reach additional patient populations that may otherwise forego surgery 65% of eligible DBS patients refuse treatment, due to fear of surgery(1) Established, Attractive Reimbursement Move procedures from the more expensive OR to the less expensive MR suite, with equivalent reimbursement Improved Utilization of Existing MRI’s 1 hour of MR Scanner time used for diagnostic imaging could generate $1,200(2) 1 hour of MR scanner time used for a ClearPoint procedure could generate $5,275(3) Utilizes existing MRI’s already in hospital (1) Medtronic Investor Presentation, June, 2014 (2) Estimated average US hospital-based MRI suite revenue per hour for outpatient diagnostic scans, based on data gathered by MRI Interventions. Excludes professional fees. Actual revenues will vary by hospital, procedure and payor. (3) Based on a weighted average payment to MRI Interventions’ customers (as of September 2014) for an electrode placement procedure for Medicare and private insurance patients, calculated by MRI Interventions using a payor mix weighted 67% to average Medicare reimbursement and 33% to average private insurance reimbursement. Average Medicare reimbursement calculated as the weighted average Medicare payment for MRI Interventions’ customers (as of September 2014) for an electrode placement procedure under MS-DRGs 025, 026 and 027. Average private insurance reimbursement calculated as 1.5x Medicare reimbursement, based on published data. Hourly amount assumes 4.5 hour procedure duration. Excludes professional fees. Actual revenues will vary by hospital, procedure and payor. © 2015 MRI INTERVENTIONS, INC. | 12 |
 |
Multi-Therapy MRI-Guided Navigational System Leading Neurosurgeon Supporters Dr. Philip Starr ASSFN Past President Dr. Paul Larson UCSF & VA Dr. Robert Gross Emory University Dr. Robert Wharen, Jr. Mayo Clinic -Jacksonville Dr. Krys Bankiewicz Bankiewicz Lab, UCSF Dr. Russ Lonser OSU -NIH Strong Peer-Reviewed Journal Support Compatible With Multiple Therapies SmartFlowTM cannula for local drug delivery © 2015 MRI INTERVENTIONS, INC. | 13 |
 |
Strong Intellectual Property Close to 100 issued patents around the world 50+ U.S. Patents 45+ OUS Patents 40+ U.S. Patent Applications 50+ OUS Patent Applications Issued patents cover areas such as: MRI-guided surgical systems that include software and devices; the SmartFrame® trajectory guide; other ClearPoint® disposable components; active intracranial probes; MRI-compatible catheters; MRI-safety technology Key ClearPoint-related patents do not begin to expire until 2027 © 2015 MRI INTERVENTIONS, INC. | 14 |
 |
Patient Impact Martin’s Story ClearPoint-Enabled Electrode Placement
© 2015 MRI INTERVENTIONS, INC. | 15 |
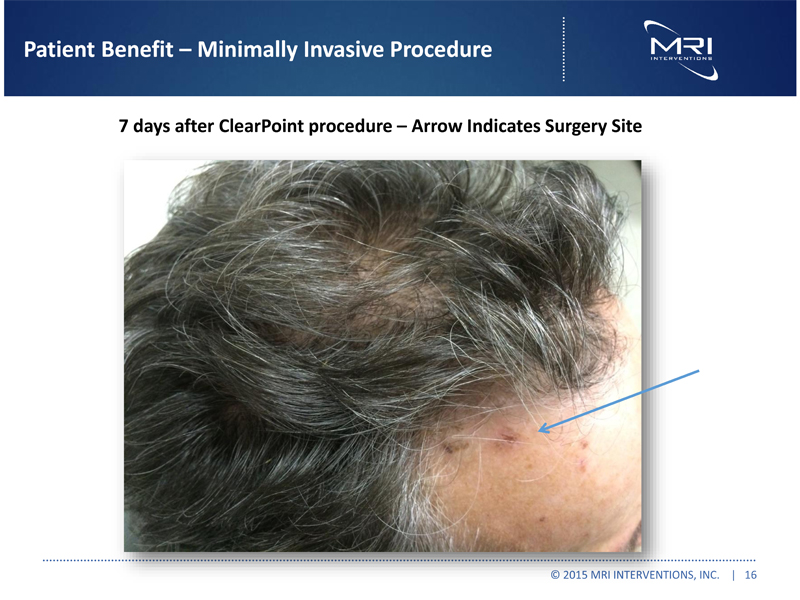 |
Patient Benefit Minimally Invasive Procedure 7 days after ClearPoint procedure Arrow Indicates Surgery Site © 2015 MRI INTERVENTIONS, INC. | 16 |
 |
ClearPoint Drug Delivery • MR visualization of neuro target • MR-guided placement of catheter • Therapeutic agent delivered under MR-guidance* Specialized drug delivery cannula’s catheters Drug infusion is visible real time under MRI Conclusion: The ClearPoint system allows Real-time Convection-enhanced Delivery to be performed with a high level of precision, predictability, and safety. *CAUTION: SmartFlowTM Cannula is approved for injection of Cytarabine or removal of CSF from the ventricles during intracranial procedures. Uses other than the approved indication are limited by Federal law to investigational use. © 2015 MRI INTERVENTIONS, INC. | 17 |
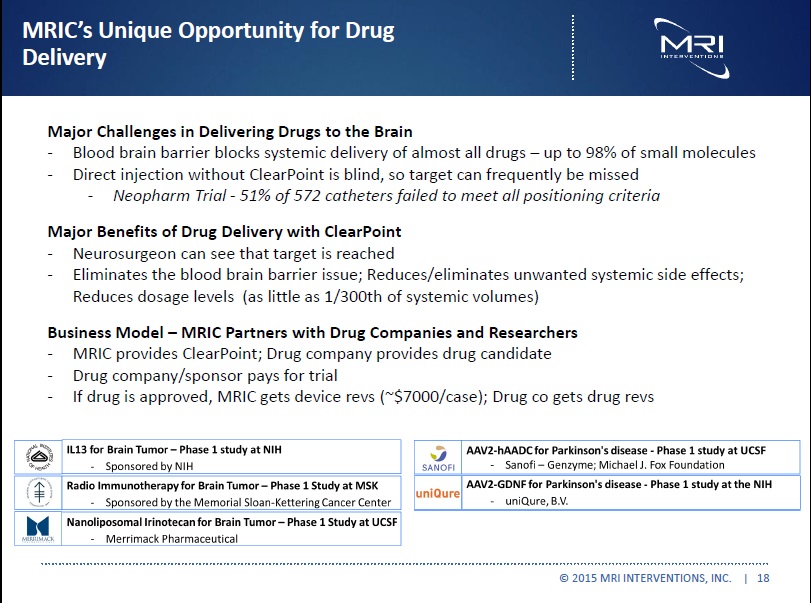 |
MRI’S Unique Opportunity in Drug Delivery Provides MRIC with “biotech-like upside” without “all or nothing downside” Major Challenges in Delivering Drugs to the Brain - Blood brain barrier blocks systemic delivery of almost all drugs – 98% of small molecules - Direct injection without ClearPoint is blind, so target is frequently missed - Neopharm Trial - 51% of 572 catheters failed to meet all positioning criteria Major Benefits of Drug Delivery with ClearPoint - Neurosurgeon sees that target is reached - Eliminates the blood brain barrier issue; Reduces/eliminates unwanted systemic side effects; Reduces dosage levels (as little as 1/300th of systemic volumes) Business Model MRIC Partners with Drug Companies and Researchers - MRIC provides ClearPoint; Drug company provides drug candidate - Drug company/sponsor pays for trial - If drug is approved, MRIC gets device revs (~$7000/case); Drug co gets drug revs
© 2015 MRI INTERVENTIONS, INC. | 18 |
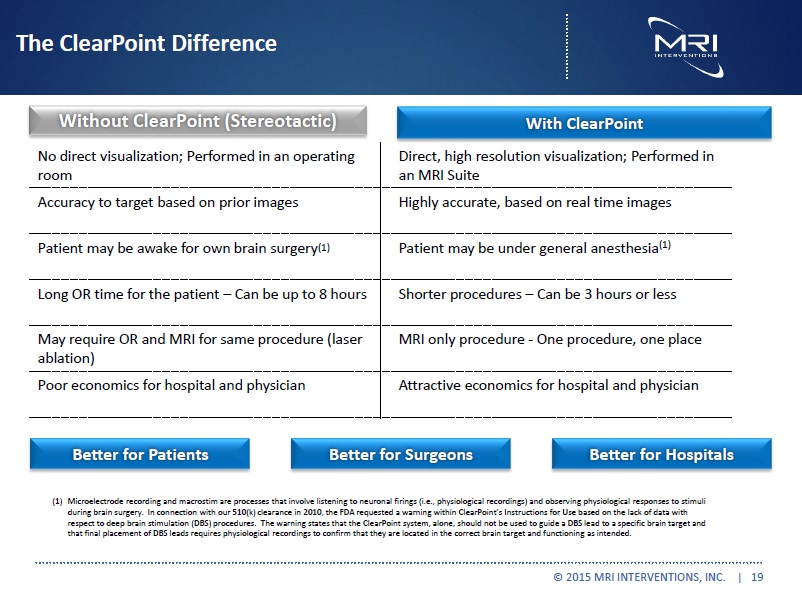 |
The ClearPoint Difference Without ClearPoint (Stereotactic) No direct visualization; Performed in anoperating room Patient may be awake forown brain surgery(1) Long procedures – Can be up to 8 hours Accuracy to target based on prior images May require OR and MRI for same procedure (laser ablation) Poor economics for hospital and physician
With ClearPoint Direct, high resolution visualization; Performed in an MRI Suite Patient may be undergeneralanesthesia(1) Shorterprocedures Can be 3 hours or less Highly accurate, based on real time images One procedure, one place Attractive economics for hospital and physician Better for Patients Better for Surgeons Better for Hospitals (1) Microelectrode recording and macrostim are processes that involve listening to neuronal firings (i.e., physiological recordings) and observing physiological responses to stimuli during brain surgery. In connections with our 510(k) clearence in 2010, the FDA requested a warning within Clearpoint’s instructions for Use based on the lack of data with respect to deep brain stimulation (DBS) procedures. The warning states that the ClearPoint system, alone, should not be used to guide a DBS lead to a specific brain target and that final placement of DBS leads requires physiological recordings to confirm that they are located in the correct brain target and functioning as intended. |
 |
ClearPoint Revenue Model BUSINESS MODEL – RAZOR / RAZORBLADE ClearPoint Hardware/Software: $100,000 - $150,000 ASP ClearPoint Disposables: $7,500 (average) ASP per procedure with strong margins Recurring revenue from the sale of disposables Procedures covered by existing inpatient DRG reimbursement codes © 2015 MRI INTERVENTIONS, INC. | 20 |
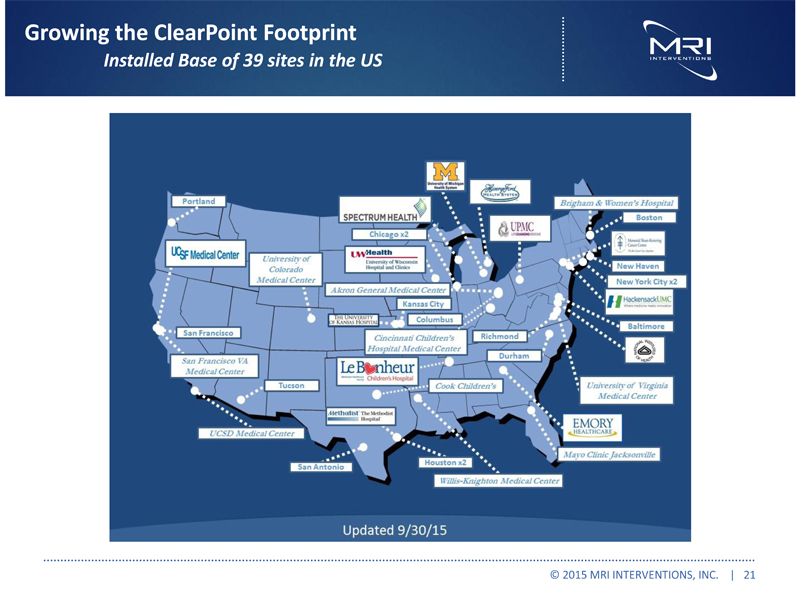 |
Growing the ClearPoint Footprint Installed Base of 39 sites in the US © 2015 MRI INTERVENTIONS, INC. | 21 |
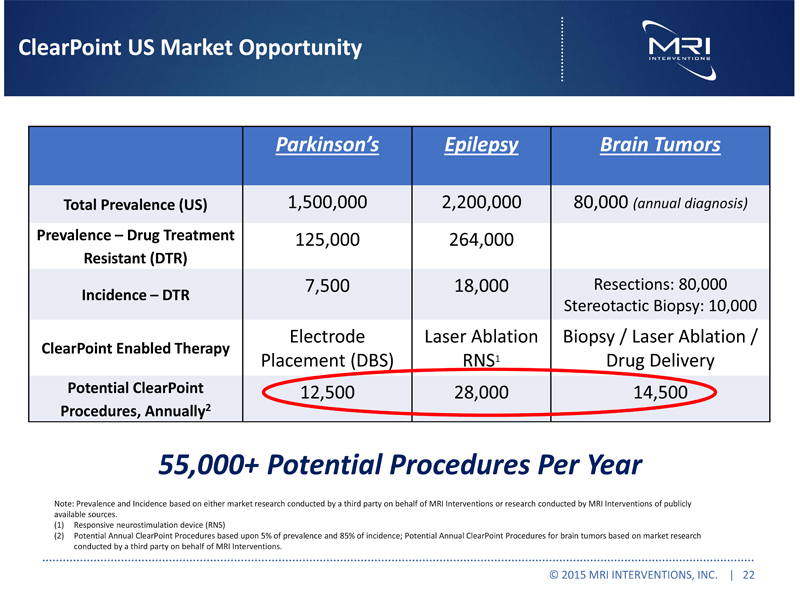 |
ClearPoint US Market Opportunity
Parkinson’s Epilepsy Brain Tumors
Total Prevalence (US) 1,500,000 2,200,000 80,000(annual diagnosis) Prevalence – Drug Treatment Resistant (DTR) 125,000 264,000 Incidence – DTR 7,500 18,000 Resections: 80,000 Stereotactic Biopsy: 10,000 ClearPoint Enabled Therapy Electrode Placement (DBS) Laser AblationRNS1 Biopsy / Laser Ablation / Drug Delivery Potential ClearPoint Procedures, Annually2 12,500 28,000 14,500 55,000+ Potential Procedures Per Year Note: Prevalence and Incidence based on either market research conducted by a third party on behalf of MRI Interventions or research conducted by MRI Interventions of publicly available sources. (1) Responsive neurostimulation device (RNS) (2) Potential Annual ClearPoint Procedures based upon 5% of prevalence and 85% of incidence; Potential Annual ClearPoint Procedures for brain tumors based on market research conducted by a third party on behalf of MRI Interventions.
© 2015 MRI INTERVENTIONS, INC. | 22 |
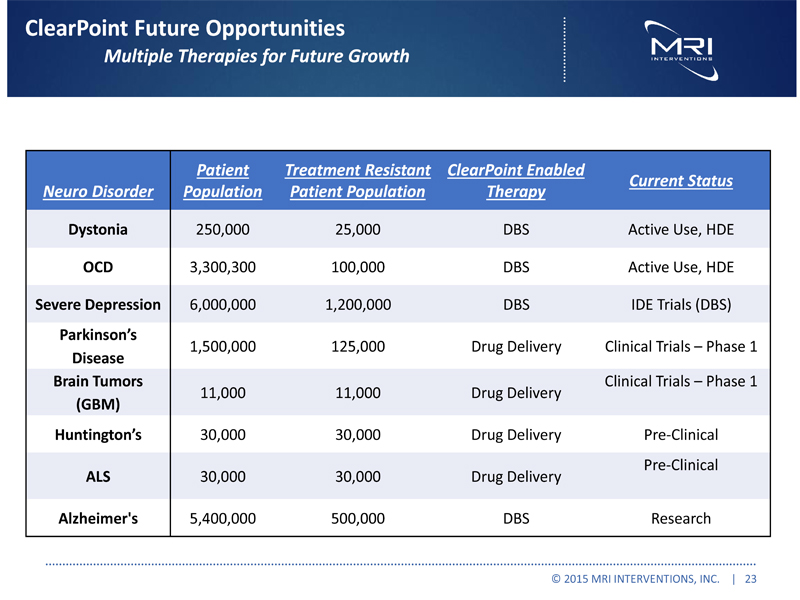 |
ClearPoint Future Opportunities Multiple Therapies for Future Growth Neuro Disorder Patient Population Treatment Resistant Patient Population ClearPoint Enabled Therapy Current Status Dystonia 250,000 25,000 DBS Active Use, HDE OCD 3,300,300 100,000 DBS Active Use, HDE Severe Depression 6,000,000 1,200,000 DBS IDE Trials (DBS) Parkinson’s Disease 1,500,000 125,000 Drug Delivery Clinical Trials – Phase 1 Brain Tumors (GBM) 11,000 11,000 Drug Delivery Clinical Trials – Phase 1 Huntington’s 30,000 30,000 Drug Delivery Pre-Clinical ALS 30,000 30,000 Drug Delivery Pre-Clinical Alzheimer's 5,400,000 500,000 DBS Research
© 2015 MRI INTERVENTIONS, INC. | 23 |
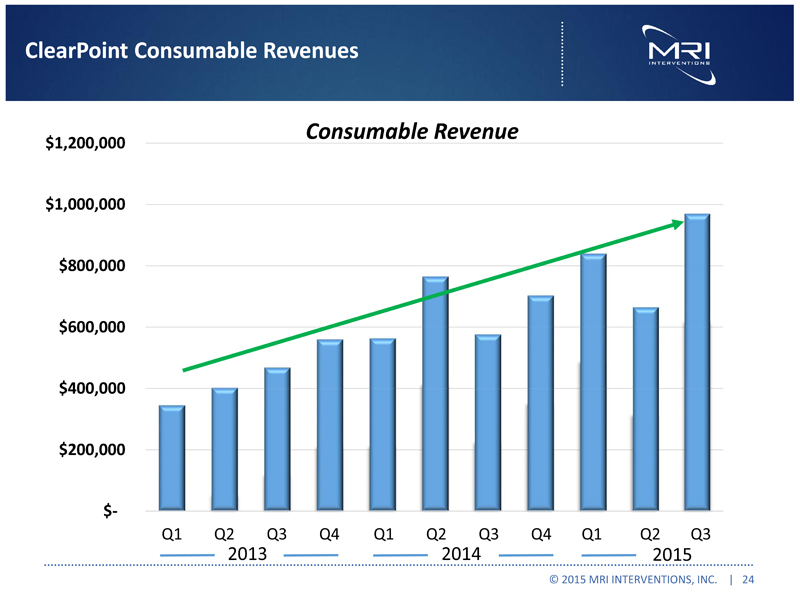 |
ClearPoint Consumable Revenues Consumable Revenue $1,200,000 $1,000,000 $800,000 $600,000 $400,000 $200,000 $- Q1 Q2 Q3 Q4 Q1 Q2 Q3 Q4 Q1 Q2 Q3 2013 2014 2015 © 2015 MRI INTERVENTIONS, INC. | 24 |
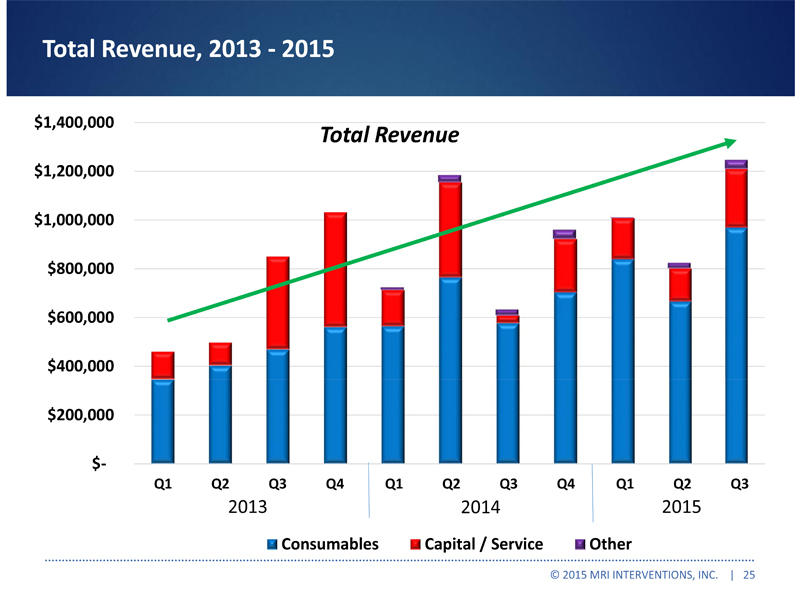 |
Total Revenue, 2013 - 2015 $1,400,000 Total Revenue $1,200,000 $1,000,000 $800,000 $600,000 $400,000 $200,000 $- Q1 Q2 Q3 Q4 Q1 Q2 Q3 Q4 Q1 Q2 Q3 2013 2014 2015 Consumables Capital / Service Other © 2015 MRI INTERVENTIONS, INC. | 25 |
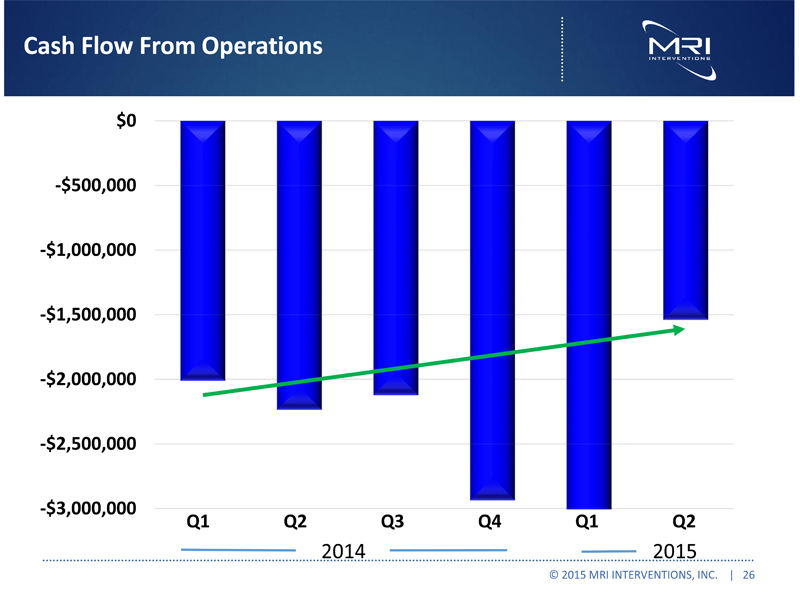 |
Cash Flow From Operations $0 -$500,000 -$1,000,000 -$1,500,000 -$2,000,000 -$2,500,000 -$3,000,000 Q1 Q2 Q3 Q4 Q1 Q2 2014 2015 © 2015 MRI INTERVENTIONS, INC. | 26 |
 |
Commercial Priorities Increase Utilization • Focus on adding surgeons at existing accounts • Target high volume sites, including epilepsy and tumor neurosurgeons within each account; gain greater share of their procedures • Add Clinical Specialists and sales reps to commercial team; compensate for utilization growth Enhance Communication • Increase peer-to-peer events, presence at trade shows • Highlight existing data on ClearPoint applications to neurologists and neurosurgeons • Communicate value proposition across procedures: • Accuracy • Real time visualization • Improved workflow • Increase patient volume
Expand Account Base • Identify highest volume potential accounts across multiple procedures • Support local hospital marketing efforts • Capitalize on interest in drug delivery to expand in oncology accounts • Add sales reps Achieve Cash Breakeven • Tightly control working capital, consumption of cash • Hire additional personnel only in key functions – commercial team; engineering
© 2015 MRI INTERVENTIONS, INC. | 27 |
 |
R&D Priorities Software 2.0 • Significant upgrade to existing software; includes real time fusion, enhanced graphic and User Interface • Technology licenses near complete with three additional software companies for this effort
OR SmartFrame • Through partnership(s), expand our products and brand into the operating room for CT based neuro procedures
Drug Delivery • Establish additional drug delivery partnerships, and participate in additional clinical trials • Become the neuro drug delivery device partner of choice
Procedure Enhancements • Continue to enhance product line with a focus on procedure simplification and consistency
Bolt Driver for Laser Ablation © 2015 MRI INTERVENTIONS, INC. | 28 |
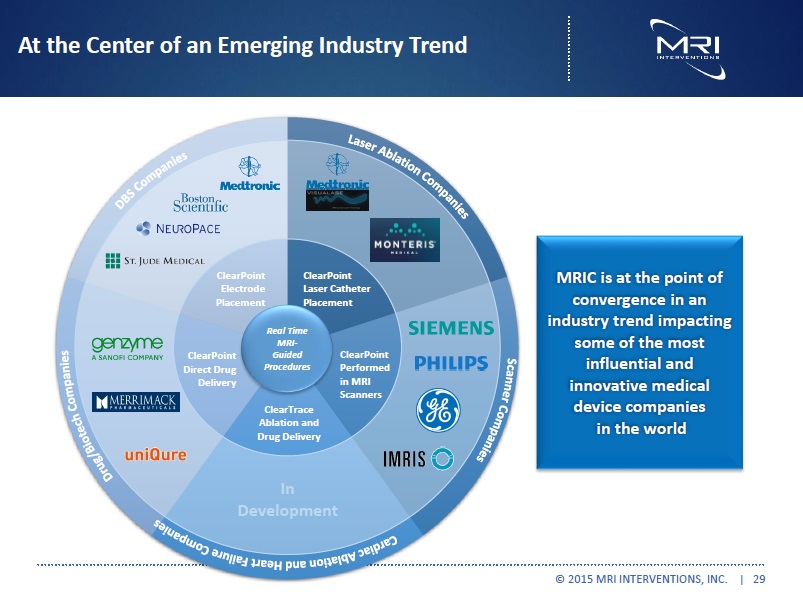 |
At the Center of an Emerging Industry Trend ClearPoint Electrode Placement ClearPoint Laser Catheter Placement ClearPoint Performed in MRI Scanners ClearTrace Ablation and Drug Delivery ClearPoint Direct Drug Delivery Real Time MRI-Guided Procedures MRIC is at the point of convergence in an industry trend impacting some of the most influential and innovative medical device companies in the world
© 2015 MRI INTERVENTIONS, INC. | 29 |
 |
Ticker: MRIC MRI Interventions, Inc. Irvine, CA 949.900.6833 mriinterventions.com Transforming minimally invasive neurosurgery by enabling real-time visualization with MRI © 2015 MRI INTERVENTIONS, INC. | 30 |