Exhibit 99.2

Ticker: MRIC Investor Presentation February 2017 Transforming minimally invasive neurosurgery by enabling real-time visualization with MRI © 2017 MRI INTERVENTIONS, INC. | 1

Forward Looking Statements Certain statements in this presentation may constitute forward-looking statements within the meaning of Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934. Forward- looking statements often can be identified by words such as “anticipates,” “believes,” “could,” “estimates,” “expects,” “intends,” “may,” “plans,” “potential,” “predicts,” “projects,” “should,” “will,” “would,” or the negative of these words or other words of similar meaning. Forward-looking statements by their nature address matters that, to different degrees, are uncertain and involve risk. Uncertainties and risks may cause MRI Interventions’ actual results and the timing of events to differ materially from those expressed in or implied by MRI Interventions’ forward-looking statements. Particular uncertainties and risks include, among others: demand and market acceptance of our products; our ability to successfully expand, and achieve full productivity from, our sales, clinical support and marketing capabilities; availability and adequacy of reimbursement from third party payors for procedures utilizing our products; the sufficiency of our cash resources to maintain planned commercialization efforts and research and development programs; future actions of the Food and Drug Administration (“FDA”) or any other regulatory body that could impact product development, manufacturing or sale; our ability to protect and enforce our intellectual property rights; our dependence on collaboration partners; the impact of competitive products and pricing; the impact of the commercial and credit environment on us and our customers and suppliers; and our ability to successfully complete the development of, and to obtain regulatory clearance or approval for, our ClearTrace system. More detailed information on these and additional factors that could affect MRI Interventions’ actual results and the timing of events are described in its filings with the Securities and Exchange Commission. Except as required by law, we undertake no obligation to publicly update or revise any forward-looking statements made in this presentation to reflect any change in our expectations or any change in events, conditions or circumstances on which any such statements are based. © 2017 MRI INTERVENTIONS, INC. | 2
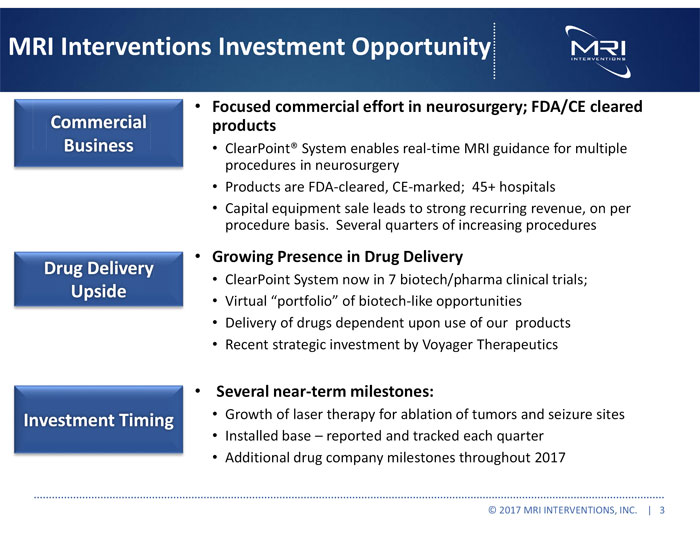
MRI Interventions Investment Opportunity Commercial Business Drug Delivery Upside Investment Timing Focused commercial effort in neurosurgery; FDA/CE cleared products ClearPoint® System enables real-time MRI guidance for multiple procedures in neurosurgery Products are FDA-cleared, CE-marked; 45+ hospitals Capital equipment sale leads to strong recurring revenue, on per procedure basis. Several quarters of increasing procedures Growing Presence in Drug Delivery ClearPoint System now in 7 biotech/pharma clinical trials; Virtual “portfolio” of biotech-like opportunities Delivery of drugs dependent upon use of our products Recent strategic investment by Voyager Therapeutics Several near-term milestones: Growth of laser therapy for ablation of tumors and seizure sites Installed base – reported and tracked each quarter Additional drug company milestones throughout 2017 © 2017 MRI INTERVENTIONS, INC. | 3
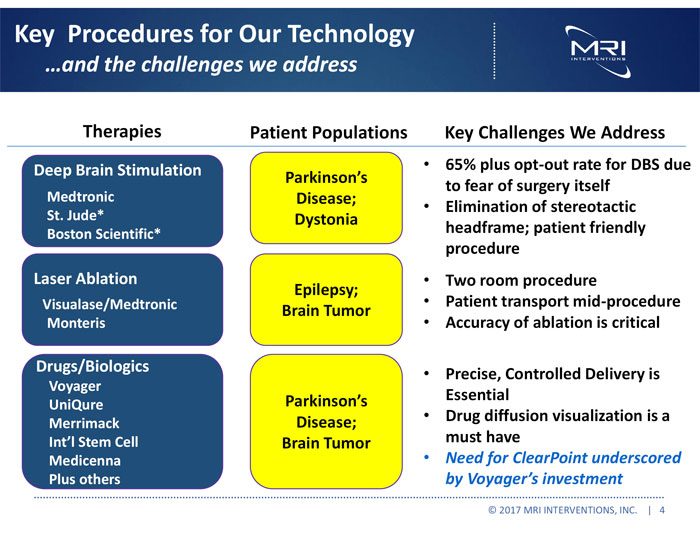
Key Procedures for Our Technology and the challenges we address Therapies Patient Populations Key Challenges We Address Deep Brain Stimulation Medtronic St. Jude* Boston Scientific* Laser Ablation Visualase/Medtronic Monteris Drugs/Biologics Voyager UniQure Merrimack Int’l Stem Cell Medicenna Plus others Parkinson’s Disease; Dystonia Epilepsy; Brain Tumor Parkinson’s Disease; Brain Tumor • 65% plus opt-out rate for DBS due to fear of surgery itself • Elimination of stereotactic headframe; patient friendly procedure • Two room procedure • Patient transport mid-procedure • Accuracy of ablation is critical • Precise, Controlled Delivery is Essential • Drug diffusion visualization is a must have • Need for ClearPoint underscored by Voyager’s investment © 2017 MRI INTERVENTIONS, INC. | 4
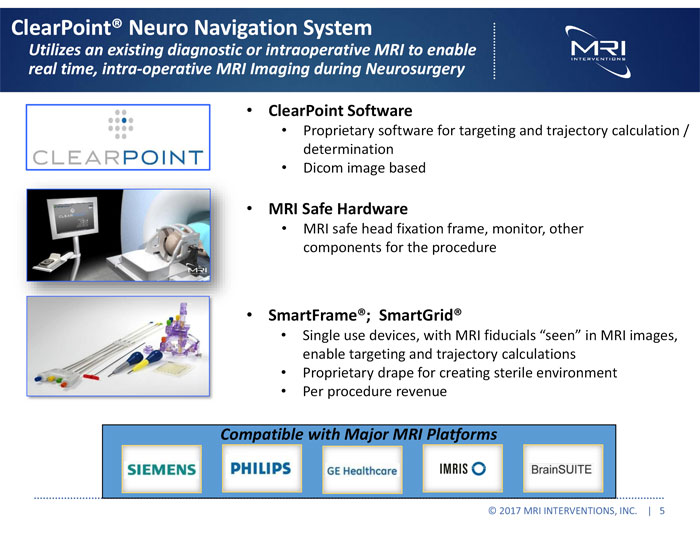
ClearPoint® Neuro Navigation System Utilizes an existing diagnostic or intraoperative MRI to enable real time, intra-operative MRI Imaging during Neurosurgery • ClearPoint Software Proprietary software for targeting and trajectory calculation / determination Dicom image based MRI Safe Hardware MRI safe head fixation frame, monitor, other components for the procedure SmartFrame®; SmartGrid® Single use devices, with MRI fiducials “seen” in MRI images, enable targeting and trajectory calculations Proprietary drape for creating sterile environment Per procedure revenue Compatible with Major MRI Platforms © 2017 MRI INTERVENTIONS, INC. | 5
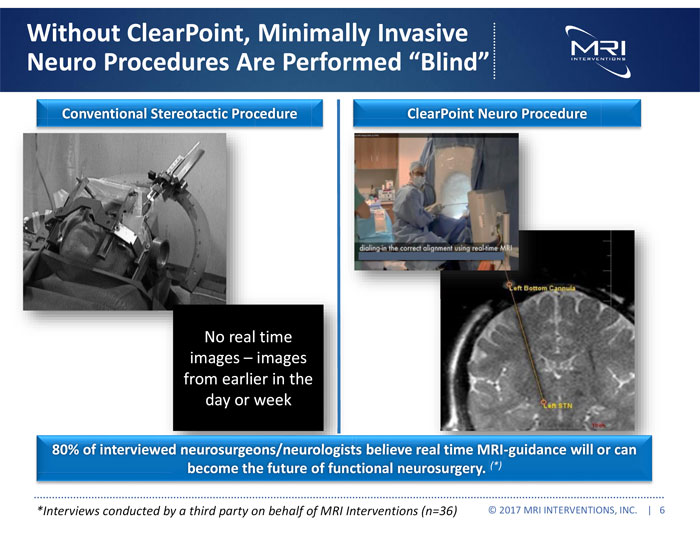
Without ClearPoint, Minimally Invasive Neuro Procedures Are Performed “Blind” Conventional Stereotactic Procedure ClearPoint Neuro Procedure No real time images – images from earlier in the day or week 80% of interviewed neurosurgeons/neurologists believe real time MRI-guidance will or can become the future of functional neurosurgery. (*) *Interviews conducted by a third party on behalf of MRI Interventions (n=36) © 2017 MRI INTERVENTIONS, INC. | 6
ClearPoint Procedure Overview Target Selection & Entry Planning VIDEO © 2017 MRI INTERVENTIONS, INC. | 7

ClearPoint Procedure Overview SmartFrame Trajectory Guide SmartFrame Hand Controller Trajectory Alignment & Device Insertion VIDEO © 2017 MRI INTERVENTIONS, INC. | 8

Clinical Support, Validation Notable Neurosurgeon Supporters Dr. Philip Starr ASSFN Past President Dr. Paul Larson UCSF & VA Dr. Robert Gross Emory University Dr. Robert Wharen, Jr. Mayo Clinic - Jacksonville Dr. Krys Bankiewicz Bankiewicz Lab, UCSF Dr. Russ Lonser OSU - NIH Published Peer-Reviewed Journal Support © 2017 MRI INTERVENTIONS, INC. | 9
Patented Intellectual Property Over 100 issued patents around the world 70+ U.S. Patents 45+ OUS Patents 20+ U.S. Patent Applications 30+ OUS Patent Applications Issued patents cover areas such as: MRI-guided surgical systems that include software and devices; the SmartFrame trajectory guide; other ClearPoint disposable components; active intracranial probes; MRI-compatible catheters and Hand Drill; MRI-safety technology; Scalp Mount Base Key ClearPoint-related patents do not begin to expire until 2027 © 2017 MRI INTERVENTIONS, INC. | 10
MRIC’s Unique Opportunity in Drug Delivery Major Challenges in Delivering Drugs to the Brain - Blood brain barrier blocks systemic delivery (pills, shots, IV) of almost all drugs – 98% of small molecules - Direct injection without ClearPoint is blind, so target is frequently missed - Neopharm Trial - 51% of 572 catheters failed to meet all positioning criteria Major Benefits of Drug Delivery with ClearPoint - Eliminates the blood brain barrier issue - Neurosurgeon sees that target is reached - Surgeon can watch drug infusion real time, is able to see appropriate coverage - Reduces/eliminates unwanted systemic side effects - Reduces dosage levels (as little as 1/300th of systemic volume) Business Model – MRIC Partners with Drug Companies and Researchers - MRIC provides ClearPoint; Drug company provides drug candidate - Drug company/sponsor pays for trial - If drug is approved, MRIC gets device revs (~$7,000 - $14,000/case); Drug co gets drug revenues Provides MRIC with “biotech-like upside” without “all or nothing downside” © 2017 MRI INTERVENTIONS, INC. | 11
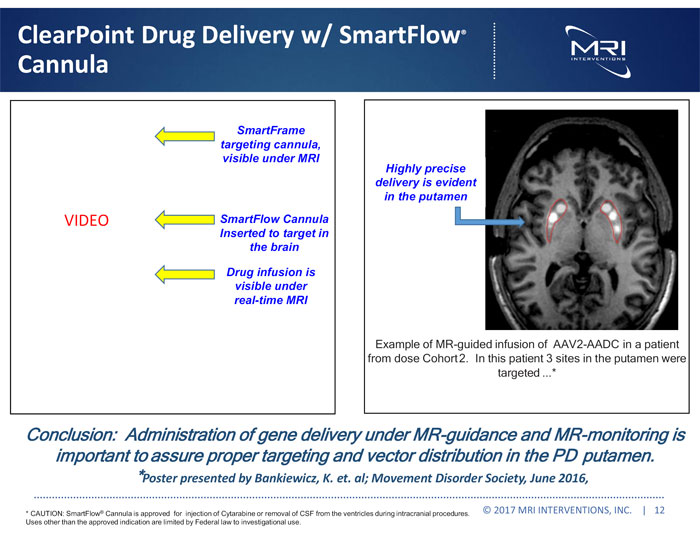
ClearPoint Drug Delivery w/ SmartFlow® Cannula VIDEO SmartFrame targeting cannula, visible under MRI SmartFlow Cannula Inserted to target in the brain Highly precise delivery is evident in the putamen Drug infusion is visible under real-time MRI Example of MR-guided infusion of AAV2-AADC in a patient from dose Cohort 2. In this patient 3 sites in the putamen were targeted ...* Conclusion: Administration of gene delivery under MR-guidance and MR-monitoring is important to assure proper targeting and vector distribution in the PD putamen. *Poster presented by Bankiewicz, K. et. al; Movement Disorder Society, June 2016, * CAUTION: SmartFlow® Cannula is approved for injection of Cytarabine or removal of CSF from the ventricles during intracranial procedures. Uses other than the approved indication are limited by Federal law to investigational use. © 2017 MRI INTERVENTIONS, INC. | 12

ClearPoint Drug Delivery w/ SmartFlow Cannula MR visualization of neuro target MR-guided placement of catheter Therapeutic agent delivered under MR-guidance* Growing Set of Peer-reviewed Publications… Specialized, FDA-cleared drug delivery cannula’s / catheters Conclusion: The ClearPoint system allows Real-time Convection- enhanced Delivery to be performed with a high level of precision, predictability, and safety. CAUTION: SmartFlow Cannula is approved for injection of Cytarabine or removal of CSF from the ventricles during intracranial procedures. Uses other than the approved indication are limited by Federal law to investigational use.© 2017 MRI INTERVENTIONS, INC. | 13
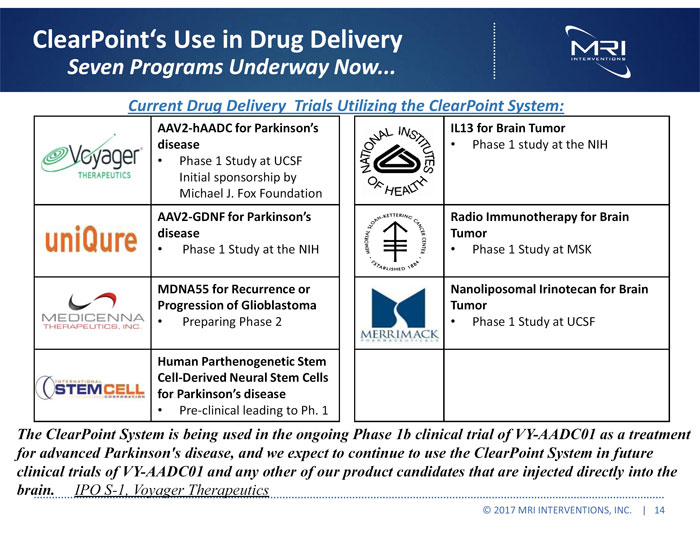
ClearPoint Use in Drug Delivery Seven Programs Underway Now Current Drug Delivery Trials Utilizing the ClearPoint System: AAV2-hAADC for Parkinson’s disease Phase 1 Study at UCSF Initial sponsorship by Michael J. Fox Foundation AAV2-GDNF for Parkinson’s disease Phase 1 Study at the NIH MDNA55 for Recurrence or Progression of Glioblastoma Preparing Phase 2 Human Parthenogenetic Stem Cell-Derived Neural Stem Cells for Parkinson’s disease Pre-clinical leading to Ph. 1 IL13 for Brain Tumor Phase 1 study at the NIH Radio Immunotherapy for Brain Tumor Phase 1 Study at MSK Nanoliposomal Irinotecan for Brain Tumor Phase 1 Study at UCSF The ClearPoint System is being used in the ongoing Phase 1b clinical trial of VY-AADC01 as a treatment for advanced Parkinson’s disease, and we expect to continue to use the ClearPoint System in future clinical trials of VY-AADC01 and any other of our product candidates that are injected directly into the brain. IPO S-1, Voyager Therapeutics © 2017 MRI INTERVENTIONS, INC. | 14

ClearPoint Revenue Model BUSINESS MODEL RAZOR / RAZORBLADE ClearPoint Hardware/Software: $100,000 - $150,000 ASP ClearPoint Disposables: $7,500 (average) ASP per procedure with potentially strong margins Recurring revenue from the sale of disposables Procedures covered by existing inpatient DRG reimbursement codes © 2017 MRI INTERVENTIONS, INC. | 15
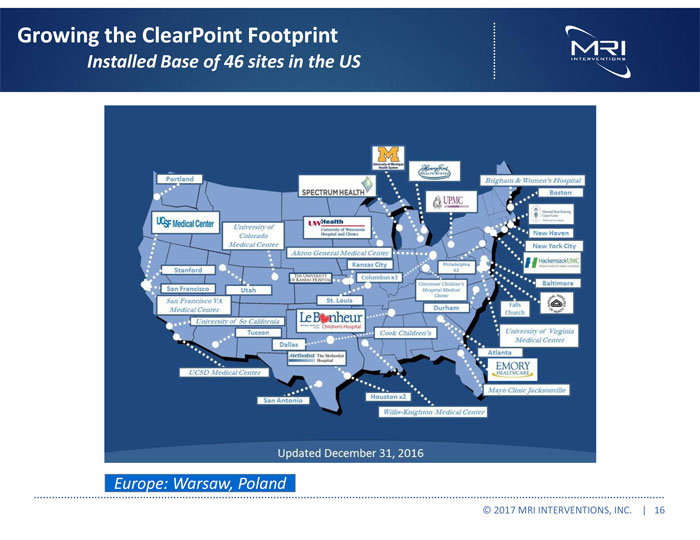
Growing the ClearPoint Footprint Installed Base of 46 sites in the US Europe: Warsaw, Poland © 2017 MRI INTERVENTIONS, INC. | 16
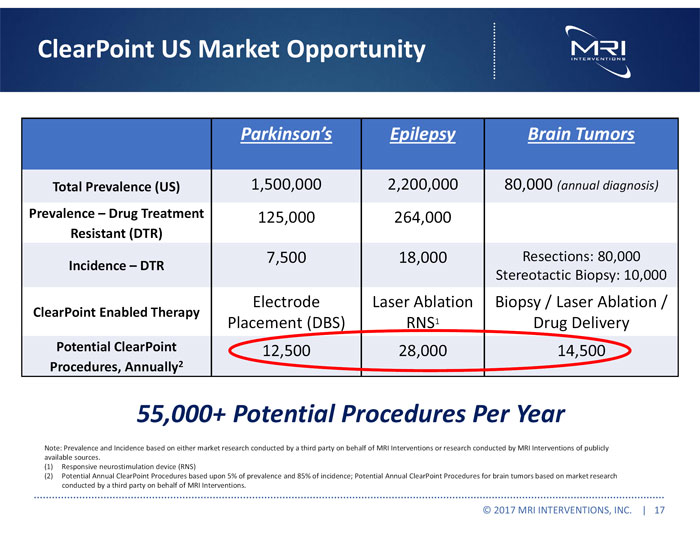
ClearPoint US Market Opportunity Parkinson’s Epilepsy Brain Tumors Total Prevalence (US) 1,500,000 2,200,000 80,000 (annual diagnosis) Prevalence – Drug Treatment Resistant (DTR) 125,000 264,000 Incidence – DTR 7,500 18,000 Resections: 80,000 Stereotactic Biopsy: 10,000 ClearPoint Enabled Therapy Electrode Placement (DBS) Laser Ablation RNS1 Biopsy / Laser Ablation / Drug Delivery Potential ClearPoint Procedures, Annually2 12,500 28,000 14,500 55,000+ Potential Procedures Per Year Note: Prevalence and Incidence based on either market research conducted by a third party on behalf of MRI Interventions or research conducted by MRI Interventions of publicly available sources. (1) Responsive neurostimulation device (RNS) (2) Potential Annual ClearPoint Procedures based upon 5% of prevalence and 85% of incidence; Potential Annual ClearPoint Procedures for brain tumors based on market research conducted by a third party on behalf of MRI Interventions. © 2017 MRI INTERVENTIONS, INC. | 17
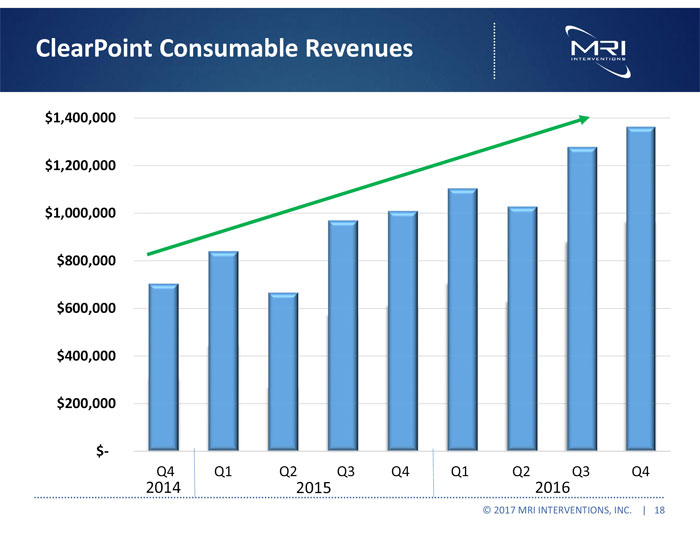
ClearPoint Consumable Revenues $1,400,000 $1,200,000 $1,000,000 $800,000 $600,000 $400,000 $200,000 $- Q4 2014 Ql Q2 Q3 Q4 2015 Ql Q2 Q3 Q4 2016 © 2017 MRI INTERVENTIONS, INC. | 18
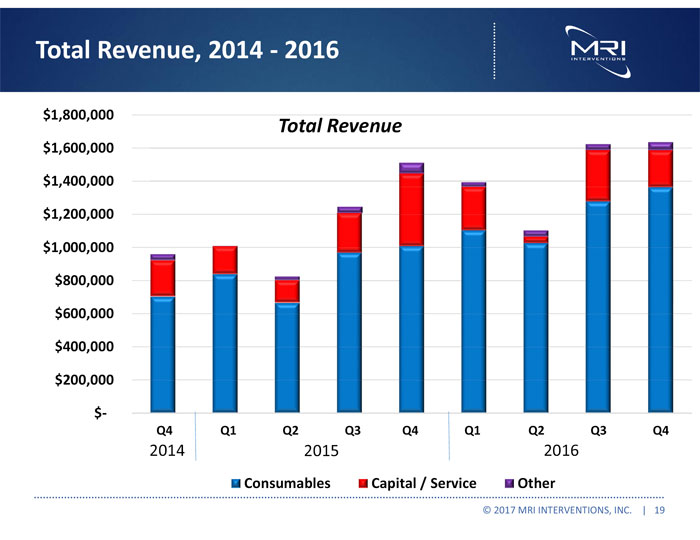
Total Revenue, 2014 - 2016 $1,800,000 $1,600,000 Total Revenue $1,400,000 $1,200,000 $1,000,000 $800,000 $600,000 $400,000 $200,000 $- Q4 Q1 Q2 Q3 Q4 Q1 Q2 Q3 Q4 2014 2015 2016 Consumables Capital / Service Other © 2017 MRI INTERVENTIONS, INC. | 19
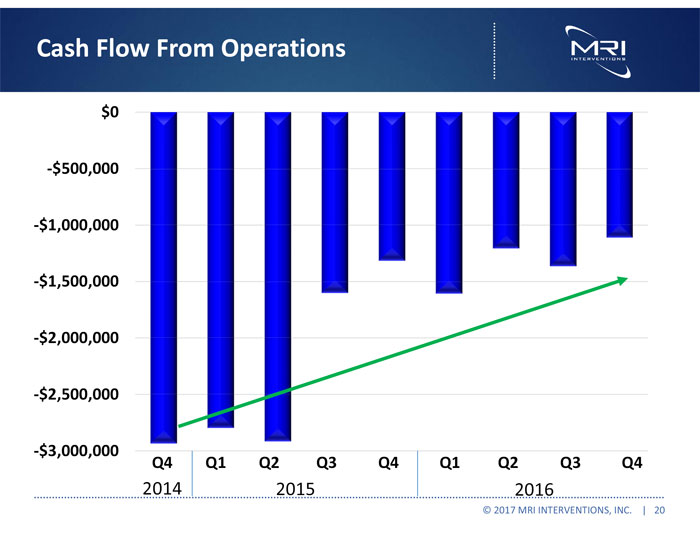
Cash Flow From Operations $0 -$500,000 -$1,000,000 -$1,500,000 -$2,000,000 -$2,500,000 -$3,000,000 Q4 2014 Q1 Q2 Q3 Q4 2015 Q1 Q2 Q3 Q4 2016 © 2017 MRI INTERVENTIONS, INC. | 20
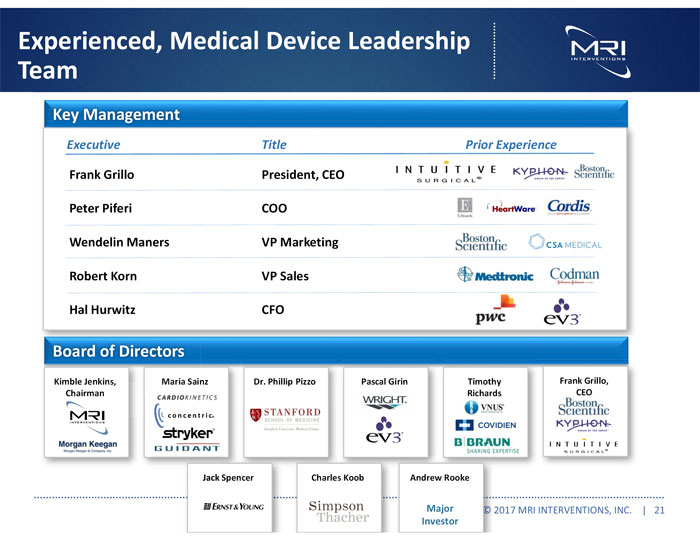
Experienced, Medical Device Leadership Team Key Management Executive Title Prior Experience Frank Grillo President, CEO Peter Piferi COO Wendelin Maners VP Marketing Robert Korn VP Sales Hal Hurwitz CFO Board of Directors Kimble Jenkins, Chairman Maria Sainz Dr. Phillip Pizzo Pascal Girin Timothy Richards Frank Grillo, CEO Jack Spencer Charles Koob Andrew Rooke Major Investor © 2017 MRI INTERVENTIONS, INC. | 21
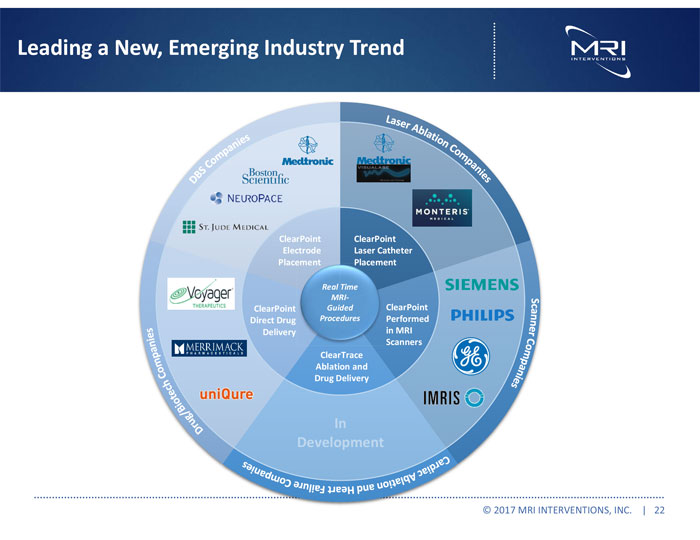
Leading a New, Emerging Industry Trend © 2017 MRI INTERVENTIONS, INC. | 22
Ticker: MRIC MRI Interventions, Inc. Irvine, CA 949.900.6833 www.mriinterventions.com Transforming minimally invasive neurosurgery by enabling real-time visualization with MRI © 2017 MRI INTERVENTIONS, INC. | 23