
Exhibit 99.1

Investor Presentation May 2017 © 2017 MRI INTERVENTIONS, INC. | 1
Forward Looking Statements
Statements herein concerning MRI Interventions, Inc. (the “Company”) plans, growth and strategies may include forward-looking statements within the context of the federal securities laws. Statements regarding the Company’s future events, developments and future performance, as well as management’s expectations, beliefs, plans, estimates or projections relating to the future, are forward-looking statements within the meaning of these laws. Uncertainties and risks may cause the Company’s actual results to differ materially from those expressed in or implied by forward-looking statements. Particular uncertainties and risks include those relating to: the Company’s ability to obtain additional financing; estimates regarding the sufficiency of the Company’s cash resources; future revenues from sales of the Company’s ClearPoint System products; and the Company’s ability to market, commercialize and achieve broader market acceptance for the Company’s ClearPoint System products. More detailed information on these and additional factors that could affect the Company’s actual results are described in the “Risk Factors” section of the Company’s Annual Report on Form 10-K for the year ended December 31, 2016, which has been filed with the Securities and Exchange Commission, and our most recently filed quarterly report on form 10-Q. © 2017 MRI INTERVENTIONS, INC. | 2

MRIC: The Platform Company for MRI-Guided Therapies MRI Interventions: Leader in the Delivery of MRI-Guided Therapies Primary Innovator in the Field, grown out of the Advanced MR department at Johns Hopkins Extensive Intellectual Property position: 70+ issued US patents, 45 international Proven track record for conceiving, developing, commercializing and securing clinical adoption for our MRI-guided therapy platform Rapidly Growing Commercial Business; Established the Industry Platform for MRI-guided, Minimally-Invasive Neurosurgery Strong and growing clinical footprint: 48 hospitals in the US Accelerating adoption, revenue growth: 8 successive quarters of record procedures Current procedures include DBS electrode placement, laser ablation, biopsy, drug delivery MRIC’s platform integrates with products from multiple companies (med device companies, imaging companies, biotechs) Expanding Platform into Adjacent Areas to Address Additional Unmet Medical Needs Expanding platform into the stroke market; Joint Development Agmt with Mayo Clinic Expanding our capabilities, adding novel ultrasound ablation technology via Co-Dev Agmt with Acoustic MedSystems; initial market is pancreatic cancer
Leveraging our existing platform, install base and technologies to enable a rapid, cost-effective path to additional markets © 2017 MRI INTERVENTIONS, INC. | 3

Why MRI-Guided Therapies? Image-guided Therapeutic Procedures Have Become a Mainstay in U.S. Healthcare 1.2 Million Arthroscopic Procedures 1.5 million Fluoroscopic Procedures 4.0 Million Laparoscopic Procedures MRI Guided Therapeutic Procedures are the Next Step in this Trend Unique capabilities that no other imaging modality can provide High resolution, three-dimensional, continuous, no radiation MRI-Guided Procedures are Uniquely Positioned to Address Significant Unmet Medical Needs Functional neurological diseases (e.g., Parkinson’s disease, Epilepsy) Untreatable hemorrhagic stroke (e.g., intracerebral hemorrhage) Certain cancers (e.g., brain, pancreas) © 2017 MRI INTERVENTIONS, INC. | 4
Our MRI-Guided Therapy Platform is Currently Being Used to: Implant Neuro Stimulation Products from: Medtronic St. Jude Medical NeuroPace Place Laser Ablation Probes from: Medtronic-Visualase Monteris Medical Deliver Drugs and Biologics from: Voyager Medicenna Oxford Biomedica International Stem Cell Corporation MRIC Platform Runs on All Major Scanners: Siemens GE Philips IMRIS © 2017 MRI INTERVENTIONS, INC. | 5
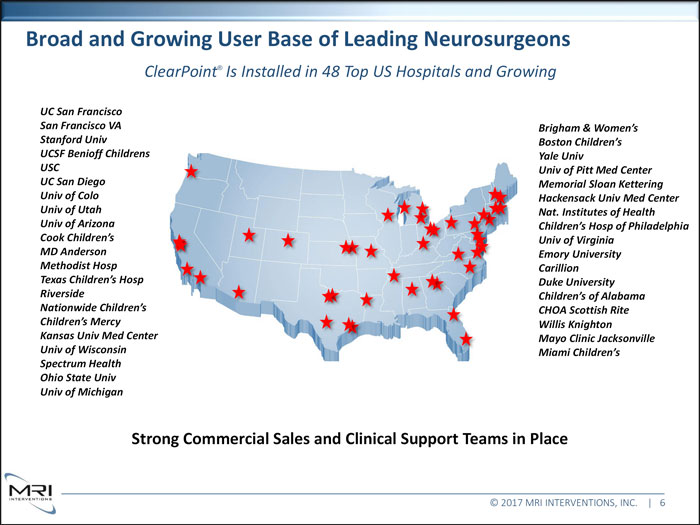
Broad and Growing User Base of Leading Neurosurgeons ClearPoint® Is Installed in 48 Top US Hospitals and Growing UC San Francisco San Francisco VA Brigham & Women’s Stanford Univ Boston Children’s UCSF Benioff Childrens Yale Univ USC Univ of Pitt Med Center UC San Diego Memorial Sloan Kettering Univ of Colo Hackensack Univ Med Center Univ of Utah Nat. Institutes of Health Univ of Arizona Children’s Hosp of Philadelphia Cook Children’s Univ of Virginia MD Anderson Emory University Methodist Hosp Carillion Texas Children’s Hosp Duke University Riverside Children’s of Alabama Nationwide Children’s CHOA Scottish Rite Children’s Mercy Willis Knighton Kansas Univ Med Center Mayo Clinic Jacksonville Univ of Wisconsin Miami Children’s Spectrum Health Ohio State Univ Univ of Michigan Strong Commercial Sales and Clinical Support Teams in Place © 2017 MRI INTERVENTIONS, INC. | 6

Foundation of Our Platform: ClearPoint Neuro Navigation System © 2017 MRI INTERVENTIONS, INC. | 7
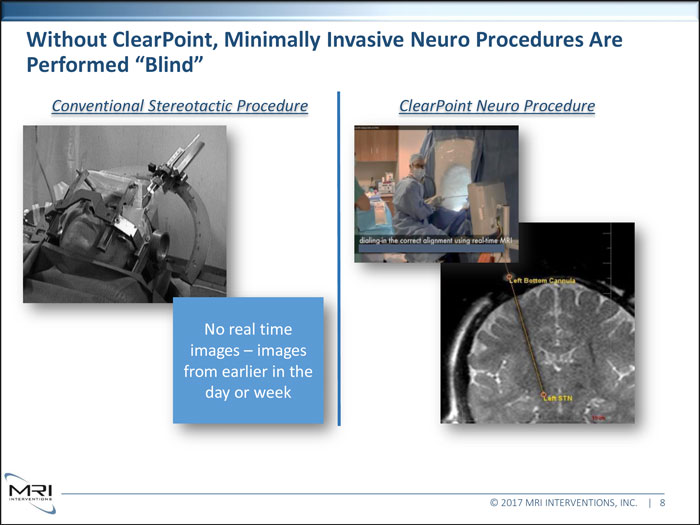
Without ClearPoint, Minimally Invasive Neuro Procedures Are Performed “Blind”Conventional Stereotactic Procedure ClearPoint Neuro ProcedureNo real time images images from earlier in the day or week © 2017 MRI INTERVENTIONS, INC. | 8
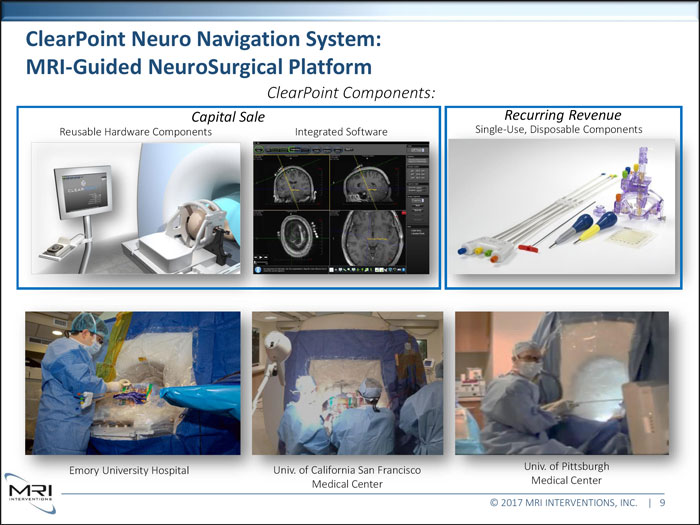
ClearPoint Neuro Navigation System: MRI-Guided NeuroSurgical Platform ClearPoint Components: Capital Sale Recurring Revenue Reusable Hardware Components Integrated Software Single-Use, Disposable Components Emory University Hospital Univ. of California San Francisco Univ. of Pittsburgh Medical Center Medical Center © 2017 MRI INTERVENTIONS, INC. | 9
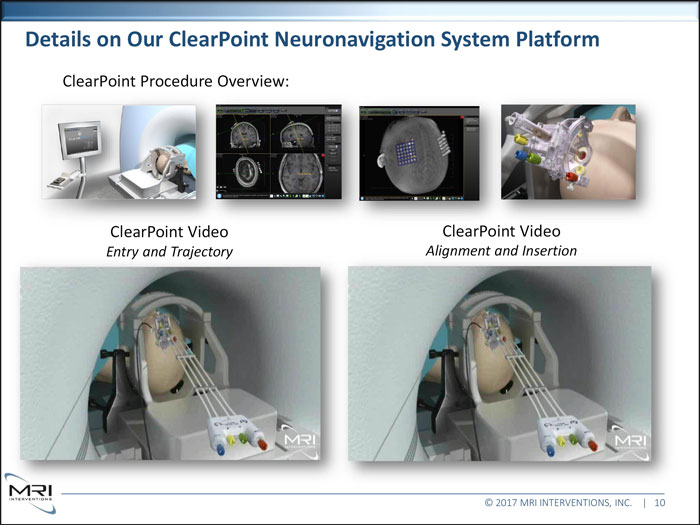
Details on Our ClearPoint Neuronavigation System Platform ClearPoint Procedure Overview: ClearPoint Video ClearPoint Video Entry and Trajectory Alignment and Insertion © 2017 MRI INTERVENTIONS, INC. | 10
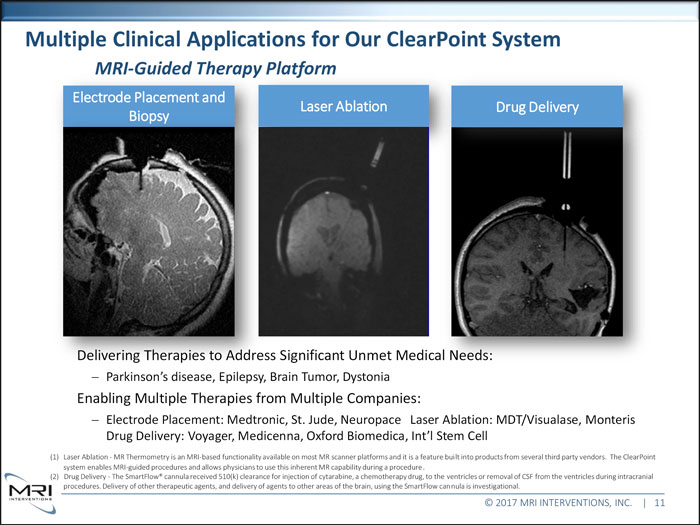
Multiple Clinical Applications for Our ClearPoint System Platform Electrode Placement and Laser Ablation Drug Delivery Delivering Therapies to Address Significant Unmet Medical Needs:Parkinson’s disease, Epilepsy, Brain Tumor, Dystonia Enabling Multiple Therapies from Multiple Companies: Electrode Placement: Medtronic, St. Jude, Neuropace Laser Ablation: MDT/Visualase, Monteris Drug Delivery: Voyager, Medicenna, Oxford Biomedica, Int’l Stem Cell (1) Laser Ablation - MR Thermometry is an MRI-based functionality available on most MR scanner platforms and it is a feature built into products from several third party vendors. The ClearPoint system enables MRI-guided procedures and allows physicians to use this inherent MR capability during a procedure. (2) Drug Delivery - The SmartFlow® cannula received 510(k) clearance for injection of cytarabine, a chemotherapy drug, to the ventricles or removal of CSF from the ventricles during intracranial procedures. Delivery of other therapeutic agents, and delivery of agents to other areas of the brain, using the SmartFlow cannula is investigational. © 2017 MRI INTERVENTIONS, INC. | 11
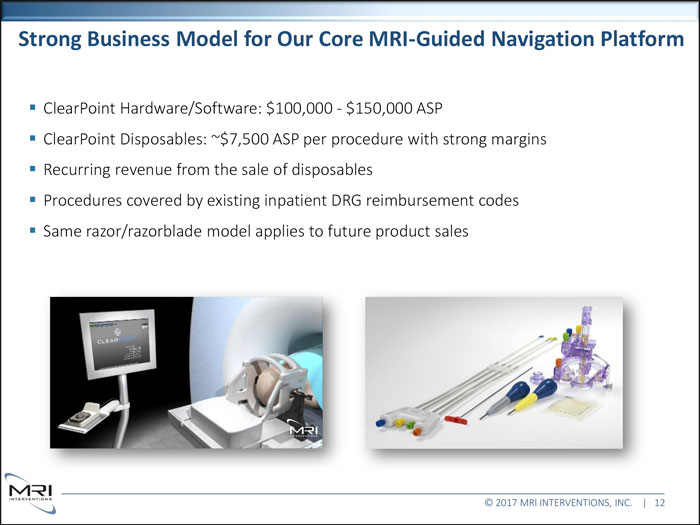
Strong Business Model for Our Core MRI-Guided Navigation Platform ClearPoint Hardware/Software: $100,000 - $150,000 ASP ClearPoint Disposables: ~$7,500 ASP per procedure with strong margins Recurring revenue from the sale of disposables Procedures covered by existing inpatient DRG reimbursement codes Same razor/razorblade model applies to future product sales © 2017 MRI INTERVENTIONS, INC. | 12
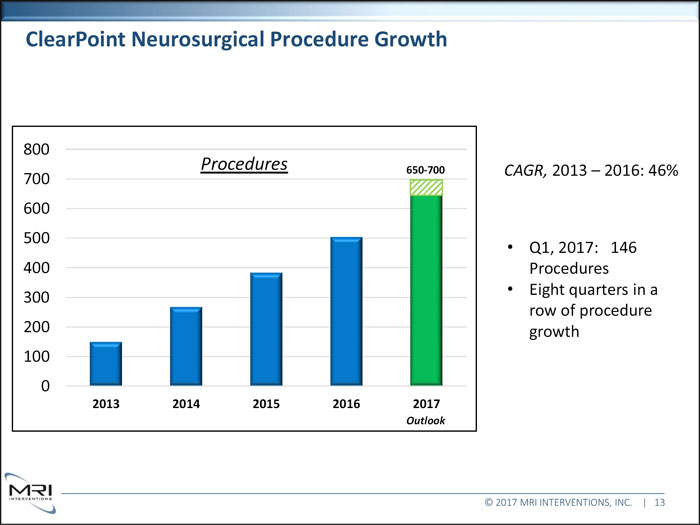
ClearPoint Neurosurgical Procedure Growth 800 Procedures 650-700 CAGR, 2013 2016: 46% 700 600 500 Q1, 2017: 146 400 Procedures Eight quarters in a 300 row of procedure 200 growth 100 0 2013 2014 2015 2016 2017 Outlook © 2017 MRI INTERVENTIONS, INC. | 13

Expanding Our Platform: New Procedural Applications © 2017 MRI INTERVENTIONS, INC. | 14
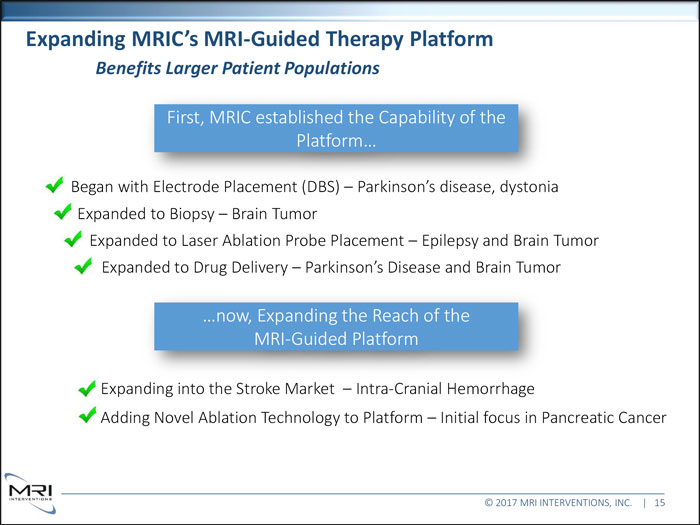
Expanding MRIC’s MRI-Guided Therapy Platform Benefits Larger Patient Populations First, MRIC established the Capability of the Platform Began with Electrode Placement (DBS) Parkinson’s disease, dystonia Expanded to Biopsy Brain Tumor Expanded to Laser Ablation Probe Placement Epilepsy and Brain Tumor Expanded to Drug Delivery Parkinson’s Disease and Brain Tumor …now, Expanding the Reach of the MRI-Guided Platform Expanding Adding Novel Ablation Technology to Platform Initial focus in Pancreatic Cancer © 2017 MRI INTERVENTIONS, INC. | 15
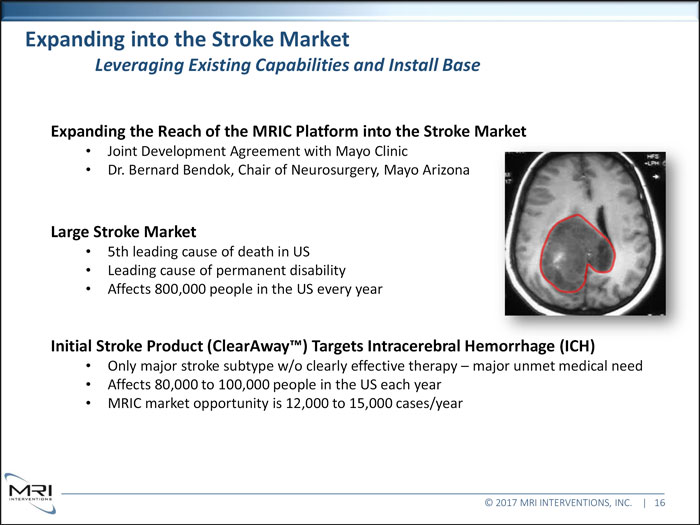
Expanding into the Stroke Market Leveraging Existing Capabilities and Install Base Expanding the Reach of the MRIC Platform into the Stroke Market Joint Development Agreement with Mayo Clinic Dr. Bernard Bendok, Chair of Neurosurgery, Mayo Arizona Large Stroke Market 5th leading cause of death in US Leading cause of permanent disability
Affects 800,000 people in the US every year Initial Stroke Product (ClearAway ) Targets Intracerebral Hemorrhage (ICH) Only major stroke subtype w/o clearly effective therapy major unmet medical need Affects 80,000 to 100,000 people in the US each year MRIC market opportunity is 12,000 to 15,000 cases/year © 2017 MRI INTERVENTIONS, INC. | 16
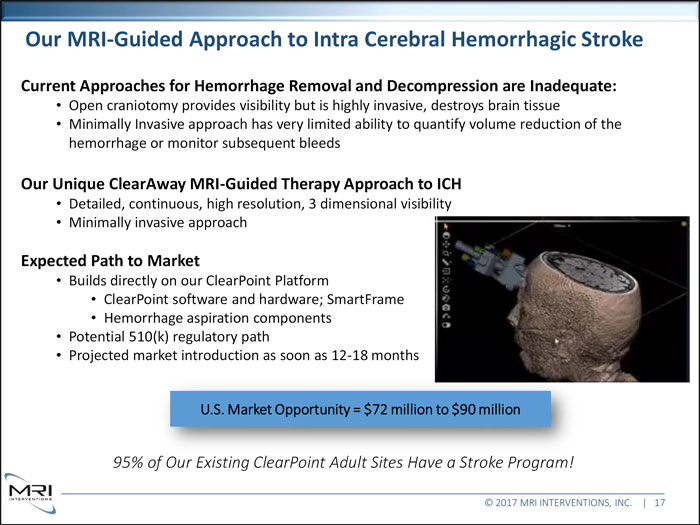
Our MRI-Guided Approach to Intra Cerebral Hemorrhagic Stroke Current Approaches for Hemorrhage Removal and Decompression are Inadequate: Open craniotomy provides visibility but is highly invasive, destroys brain tissue Minimally Invasive approach has very limited ability to quantify volume reduction of the hemorrhage or monitor subsequent bleeds Our Unique ClearAway MRI-Guided Therapy Approach to ICH Detailed, continuous, high resolution, 3 dimensional visibility Minimally invasive approach Expected Path to Market Builds directly on our ClearPoint Platform ClearPoint software and hardware; SmartFrame Hemorrhage aspiration components Potential 510(k) regulatory path Projected market introduction as soon as 12-18 months U.S. Market Opportunity = $72 million to $90 million 95% of Our Existing ClearPoint Adult Sites Have a Stroke Program! © 2017 MRI INTERVENTIONS, INC. | 17
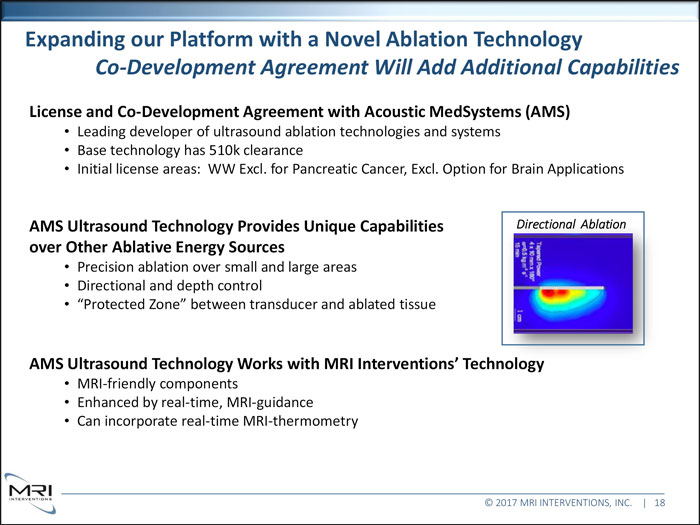
Expanding our Platform with a Novel Ablation Technology Co-Development Agreement Will Add Additional Capabilities License and Co-Development Agreement with Acoustic MedSystems (AMS) Leading developer of ultrasound ablation technologies and systems Base technology has 510k clearance Initial license areas: WW Excl. for Pancreatic Cancer, Excl. Option for Brain Applications AMS Ultrasound Technology Provides Unique Capabilities Directional Ablation over Other Ablative Energy Sources Precision ablation over small and large areas Directional and depth control “Protected Zone” between transducer and ablated tissue AMS Ultrasound Technology Works with MRI Interventions’ Technology
MRI-friendly components Enhanced by real-time, MRI-guidance Can incorporate real-time MRI-thermometry © 2017 MRI INTERVENTIONS, INC. | 18
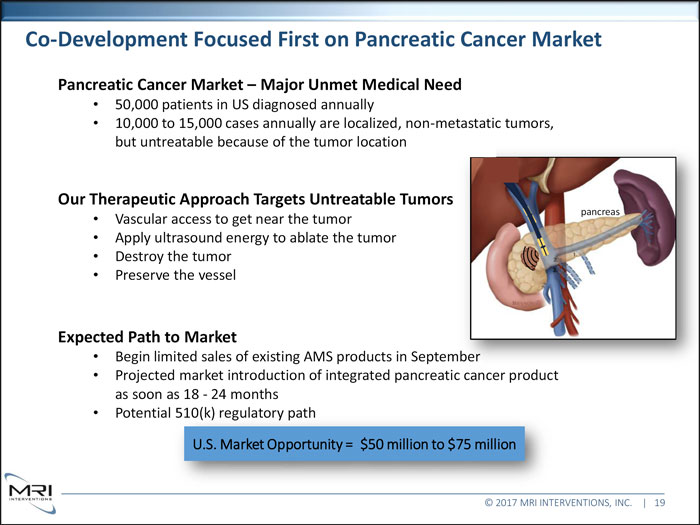
Co-Development Focused First on Pancreatic Cancer Market Pancreatic Cancer Market Major Unmet Medical Need 50,000 patients in US diagnosed annually 10,000 to 15,000 cases annually are localized, non-metastatic tumors, but untreatable because of the tumor location Our Therapeutic Approach Targets Untreatable Tumors pancreas Vascular access to get near the tumor Apply ultrasound energy to ablate the tumor Destroy the tumor Preserve the vessel Expected Path to Market Begin limited sales of existing AMS products in September
Projected market introduction of integrated pancreatic cancer product as soon as 18 - 24 months Potential 510(k) regulatory path U.S. Market Opportunity = $50 million to $75 million © 2017 MRI INTERVENTIONS, INC. | 19
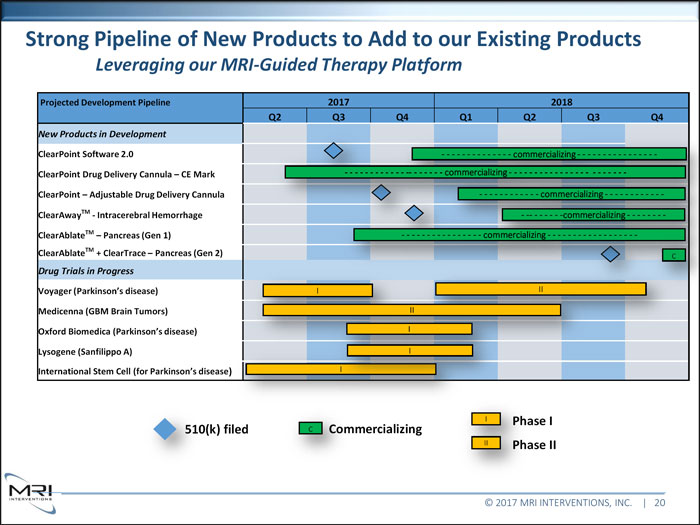
Strong Pipeline of New Products to Add to our Existing Products Leveraging our MRI-Guided Therapy Platform Projected Development Pipeline 2017 2018 Q2 Q3 Q4 Q1 Q2 Q3 Q4 New Products in Development ClearPoint Software 2.0 ClearPoint Drug Delivery Cannula CE Mark - - - - - - - - - - - - -- - - - - - - ClearPoint Adjustable Drug Delivery Cannula - -
ClearAwayTM - Intracerebral Hemorrhage ClearAblateTM Pancreas (Gen 1) - - - - - - - - - - - - - - - - commercializing - - - - - - - - - - - - - - - - - - ClearAblateTM + ClearTrace Pancreas (Gen 2) c Drug Trials in Progress Voyager (Parkinson’s disease) II Medicenna (GBM Brain Tumors) Oxford Biomedica (Parkinson’s disease) Lysogene (Sanfilippo A) I International Stem Cell (for Parkinson’s disease) I I filed c Commercializing II II © 2017 MRI INTERVENTIONS, INC. | 20
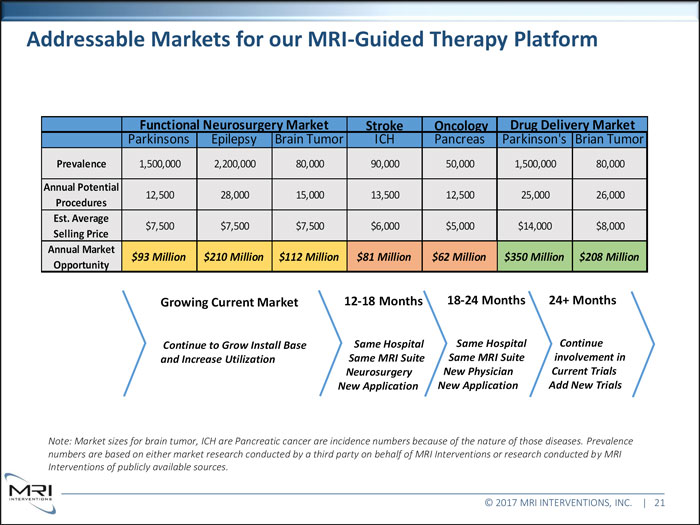
Addressable Markets for our MRI-Guided Therapy PlatformFunctional Neurosurgery Market Stroke Oncology Drug Delivery Market Parkinsons Epilepsy Brain Tumor ICH Pancreas Parkinson’s Brian Tumor Prevalence 1,500,000 2,200,000 80,000 90,000 50,000 1,500,000 80,000 Annual Potential 12,500 28,000 15,000 13,500 12,500 25,000 26,000 Procedures Est. Average $7,500 $7,500 $7,500 $6,000 $5,000 $14,000 $8,000 Selling Price Annual Market $93 Million $210 Million $112 Million $81 Million $62 Million $350 Million $208 Million
Opportunity Growing Current Market 12-18 Months 18-24 Months 24+ Months Continue to Grow Install Base Same Hospital Same Hospital Continue and Increase Utilization Same MRI Suite Same MRI Suite involvement in Neurosurgery New Physician Current Trials New Application New Application Add New Trials Note: Market sizes for brain tumor, ICH are Pancreatic cancer are incidence numbers because of the nature of those diseases. Prevalence numbers are based on either market research conducted by a third party on behalf of MRI Interventions or research conducted by MRI Interventions of publicly available sources. © 2017 MRI INTERVENTIONS, INC. | 21

Financials © 2017 MRI INTERVENTIONS, INC. | 22
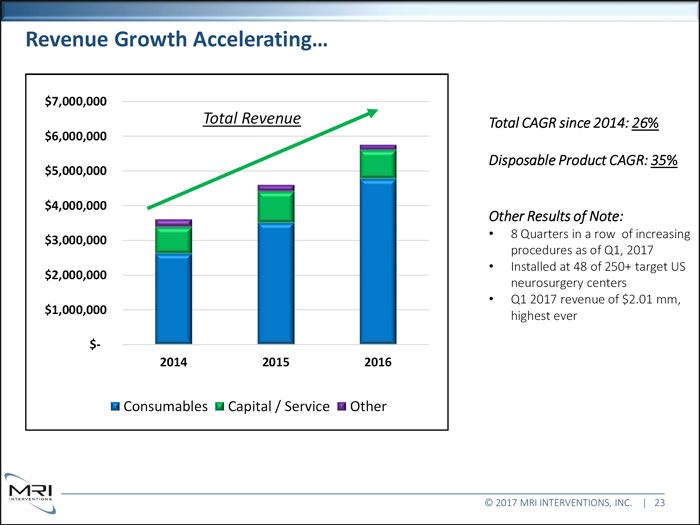
Revenue Growth Accelerating… $7,000,000 Total Revenue Total CAGR since 2014: 26% $6,000,000 Disposable Product CAGR: 35% $5,000,000 $4,000,000 Other Results of Note: 8 Quarters in a row of increasing $3,000,000 procedures as of Q1, 2017 Installed at 48 of 250+ target US $2,000,000 neurosurgery centers Q1 2017 revenue of $2.01 mm, 1,000,000 highest ever $- 2014 2015 2016 Consumables Capital / Service Other © 2017 MRI INTERVENTIONS, INC. | 23
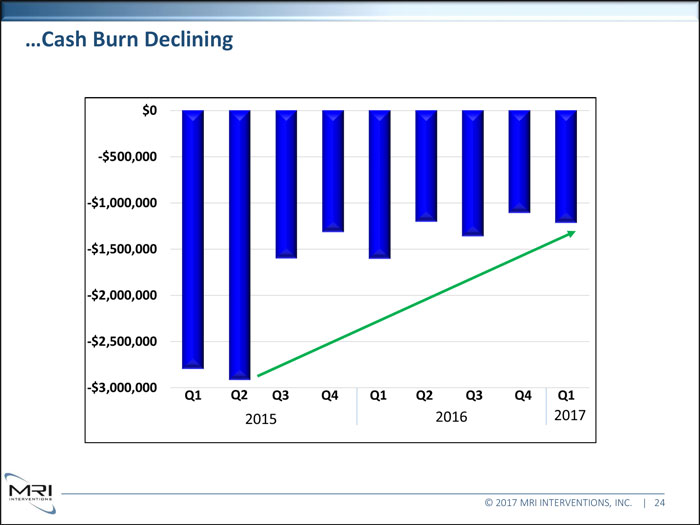
Cash Burn Declining © 2017 MRI INTERVENTIONS, INC. | 24
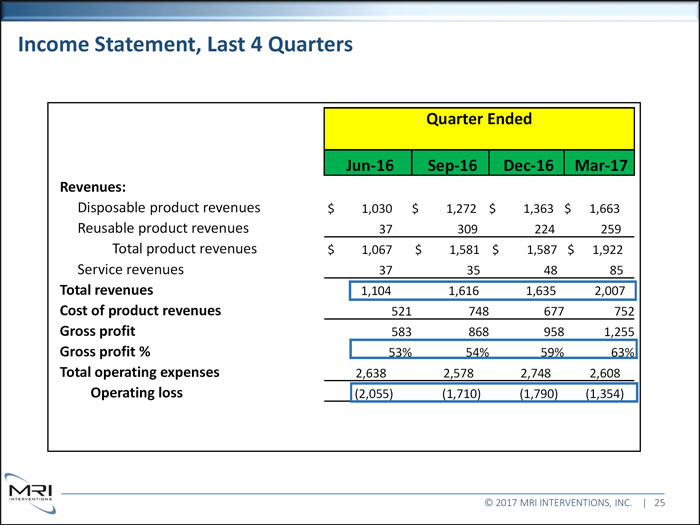
Income Statement, Last 4 Quarters Quarter Ended Jun-16 Sep-16 Dec-16 Mar-17 Revenues: Disposable product revenues $ 1,030 $ 1,272 $ 1,363 $ 1,663 Reusable product revenues 37 309 224 259 Total product revenues $ 1,067 $ 1,581 $ 1,587 $ 1,922 Service revenues 37 35 48 85 Total revenues 1,104 1,616 1,635 2,007 Cost of product revenues 521 748 677 752
Gross profit 583 868 958 1,255 Gross profit % 53% 54% 59% 63% Total operating expenses 2,638 2,578 2,748 2,608 Operating loss (2,055) (1,710) (1,790) (1,354) © 2017 MRI INTERVENTIONS, INC. | 25
Management Team Frank Grillo CEO Peter Piferi COO Wendelin Maners VP Sales & Mrktg Hal Hurwitz CFO Board of Directors Kimble Jenkins, Chairman Maria Sainz Dr. Phillip Pizzo Pascal Girin Timothy Richards Frank Grillo, CEO Spencer Charles Koob Andrew Rooke © 2017 MRI INTERVENTIONS, INC. | 1

Summary Significant Value in Owning the MRI-Guided Therapy Platform Leader in this Field Primary Innovator, Established Clinical Footprint, Industry Integration, IP World-Class Research Institutions Behind All Major Initiatives Proven Ability to Develop, Commercialize and Secure Clinical Adoption of our Platform Leveraging our Prior Investment to Cost-Effectively Expand into Stroke Market Adding a Unique Ultrasound Ablation Capability to Broaden our Platform Strong Revenue Growth and a Strong Product Pipeline © 2017 MRI INTERVENTIONS, INC. | 2