
Exhibit 99.1

Investor Presentation
Joe Burnett, President & CEO June 2018

Forward Looking Statements
Statements herein concerning MRI Interventions, Inc. (the “Company”) plans, growth and strategies may include forward-looking statements within the context of the federal securities laws. Statements regarding the Company's future events, developments and future performance, as well as management's expectations, beliefs, plans, estimates or projections relating to the future, are forward-looking statements within the meaning of these laws. Uncertainties and risks may cause the Company's actual results to differ materially from those expressed in or implied by forward-looking statements. Particular uncertainties and risks include those relating to: the Company’s ability to obtain additional financing; estimates regarding the sufficiency of the Company’s cash resources; future revenues from sales of the Company’s ClearPoint® System products; and the Company’s ability to market, commercialize and achieve broader market acceptance for the Company’s ClearPoint System products. More detailed information on these and additional factors that could affect the Company’s actual results are described in the “Risk Factors” section of the Company’s Annual Report on Form 10-K for the year ended December 31, 2017, which has been filed with the Securities and Exchange Commission, and the Company’s Quarterly Report on Form 10-Q for the quarter ended March 31, 2018, which the Company intends to file with the Securities and Exchange Commission on or before May 15, 2018.
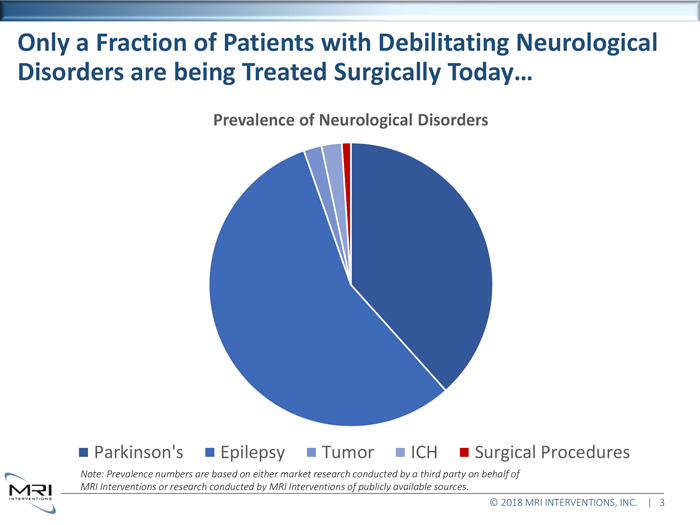
Only a Fraction of Patients with Debilitating Neurological
Disorders are being Treated Surgically Today…
Prevalence of Neurological Disorders
Parkinson's Epilepsy Tumor ICH Surgical Procedures
Note: Prevalence numbers are based on either market research conducted by a third party on behalf of
MRI Interventions or research conducted by MRI Interventions of publicly available sources.
The Movement to Minimally Invasive Procedures has
Happened Everywhere Else in the Body…
This transition has always had two things in common;
1. More patients being treated
2. Procedures are ENABLED BY LIVE IMAGE GUIDANCE
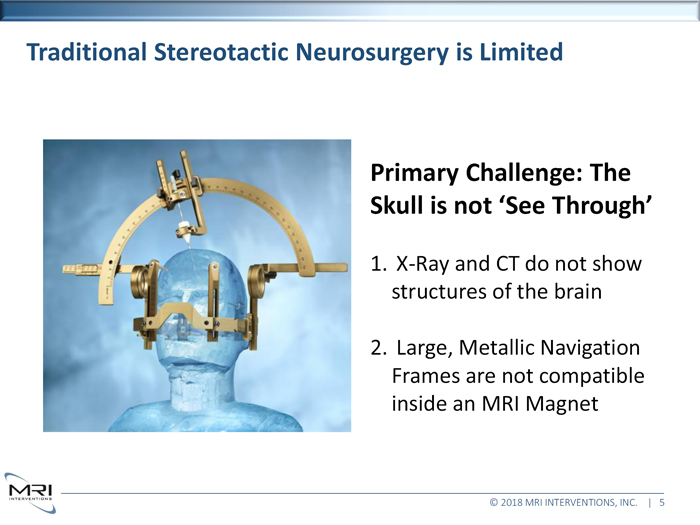
Traditional Stereotactic Neurosurgery is Limited
Primary Challenge: The
Skull in not ‘See Through’
1. X-Ray and CT do not show structures of the brain
2. Large, Metallic Navigation Frames are not compatible inside an MRI Magnet
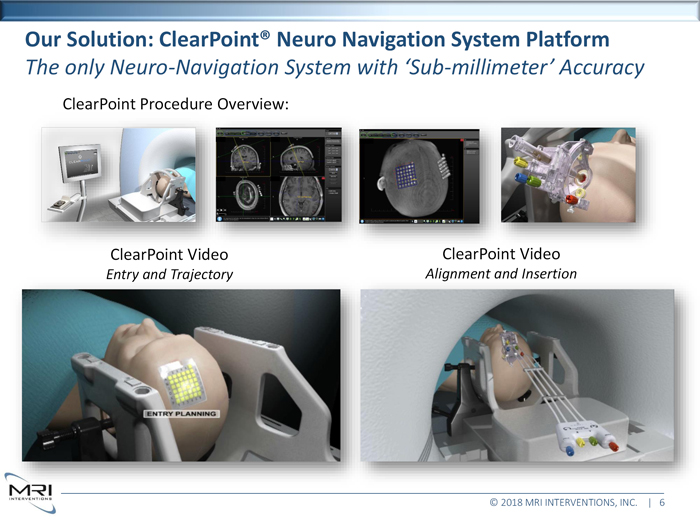
Our Solution: ClearPoint® Neuro Navigation System Platform
The only Neuro-Navigation System with ‘Sub-millimeter’ Accuracy
ClearPoint Procedure Overview:
ClearPoint Video
Entry and Trajectory
ClearPoint Video
Alignment and Insertion
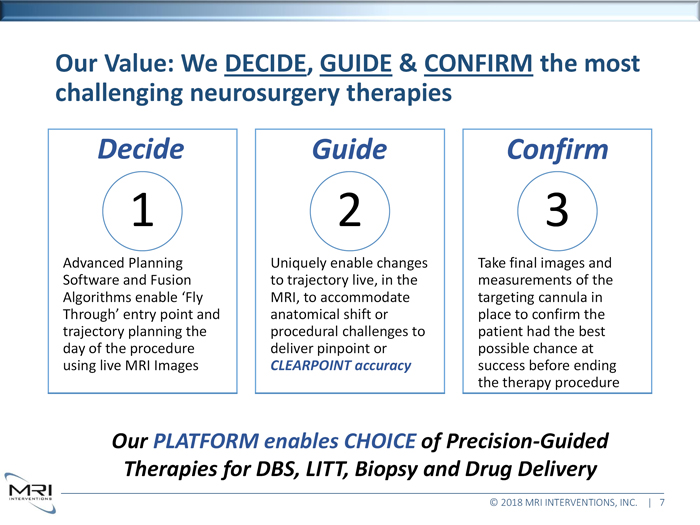
Our Value: We DECIDE, GUIDE & CONFIRM the most
challenging neurosurgery therapies Decide 1 Advanced Planning Software and Fusion Algorithms enable ‘Fly Through’ entry point and trajectory planning the day of the procedure using live MRI Images Guide 2 Uniquely enable changes to trajectory live, in the MRI, to accommodate anatomical shift or procedural challenges to deliver pinpoint or CLEARPOINT accuracy Confirm 3 Take final images and measurements of the targeting cannula in place to confirm the patient had the best possible chance at success before ending the therapy procedure
Our PLATFORM enables CHOICE of Precision-Guided
Therapies for DBS, LITT, Biopsy and Drug Delivery

MRIC: The Premier Company for Precision MRI-Guided Therapy
• MRI Interventions: Leader in Precision MRI-Guided Therapy
• Unique Platform that allows delivery of therapies to the brain with pinpoint accuracy under live MRI Guidance
• Entire procedure can take place in the MRI suite, reducing the risk of hospital acquired infections and complications
• Extensive Intellectual Property position: 70+ issued US patents, 45 international
• Procedure Growth Fueled By Expanding Installed Base and Increased Utilization
• 53 Installed systems in the U.S. at leading Neurosurgery Hospitals and Teaching Programs
• Each new system placement enables razor – razorblade disposable product sales
• Procedure count continues to grow; 43% CAGR (est.) the past four years
• Established direct sales channel totaling 16 people supporting cases in the U.S.
• Expanding Platform into Adjacent Areas to Address Additional Unmet Medical Needs
• Five current Biologics and Drug Partners using MRIC for delivery in therapy trials
• Internally developed Neuro-Aspiration device will add therapy to our sales team portfolio
• Existing and New addressable markets > $1B in potential
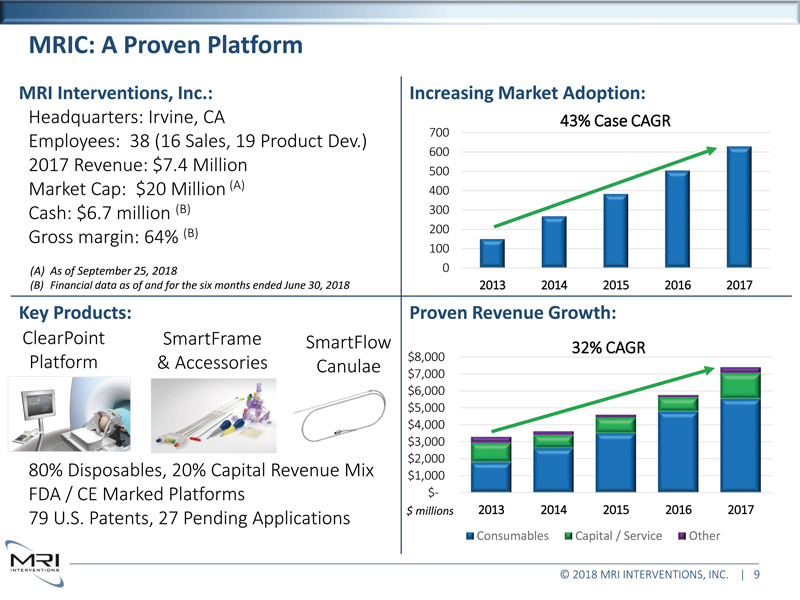
MRIC: A Proven Platform MRI Interventions, Inc.: Headquarters: Irvine, CA Employees: 39 (16 Sales, 14 Product Dev.) 2017 Revenue: $7.4 Million Market Cap: $23 Million Cash: $7.5 million Gross margin: 63.7% Financial data as of March 31, 2018 quarter end Share data as of June 1, 2018 Key Products: ClearPoint SmartFrame SmartFlow Platform & Accessories Canulae Mix FDA / CE Marked Platforms 79 U.S. Patents, 27 Pending Applications Increasing Market Adoption: 43% Case CAGR 700 600 500 400 300 200 100 0 2013 2014 2015 2016 2017 Proven Revenue Growth: 32% CAGR $8,000 $7,000 $6,000 $5,000 $4,000 $3,000 $2,000 $1,000 $ millions $- 2013 2014 2015 2016 2017
Consumables Capital / Service Other © 2018 MRI INTERVENTIONS, INC. | 9
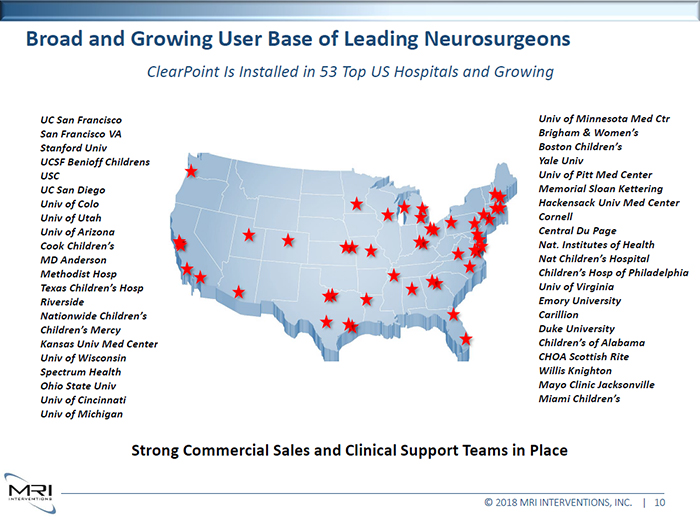
Broad and Growing User Base of Leading Neurosurgeons ClearPoint Is Installed in 53 Top US Hospitals and Growing UC San Francisco San Francisco VA Stanford Univ UCSF Benioff Childrens USC
UC San Diego Univ of Colo Univ of Utah Univ of Arizona Cook Children’s MD Anderson Methodist Hosp Texas Children’s Hosp Riverside Nationwide Children’s Children’s Mercy Kansas Univ Med Center Univ of Wisconsin Spectrum Health Ohio State Univ Univ of Cincinnati Univ of Michigan Univ of Minnesota Med Ctr Brigham & Women’s Boston Children’s Yale Univ Univ of Pitt Med Center Memorial Sloan Kettering Hackensack Univ Med Center Cornell Central Du Page Nat. Institutes of Health Nat Children’s Hospital Children’s Hosp of Philadelphia Univ of Virginia Emory University Carillion Duke University Children’s of Alabama CHOA Scottish Rite Willis Knighton Mayo Clinic Jacksonville Miami Children’s Strong Commercial Sales and Clinical Support Teams in Place
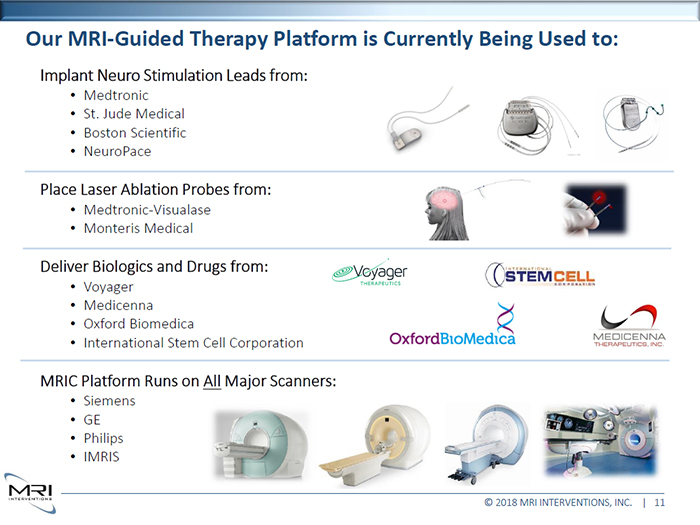
Our MRI-Guided Therapy Platform is Currently Being Used to: Implant Neuro Stimulation Leads from: Medtronic • St. Jude Medical • Boston Scientific • NeuroPace Place Laser Ablation Probes from: • Medtronic-Visualase • Monteris Medical MRIC Platform Runs on All Major Scanners: • Siemens • GE • Philips • IMRIS
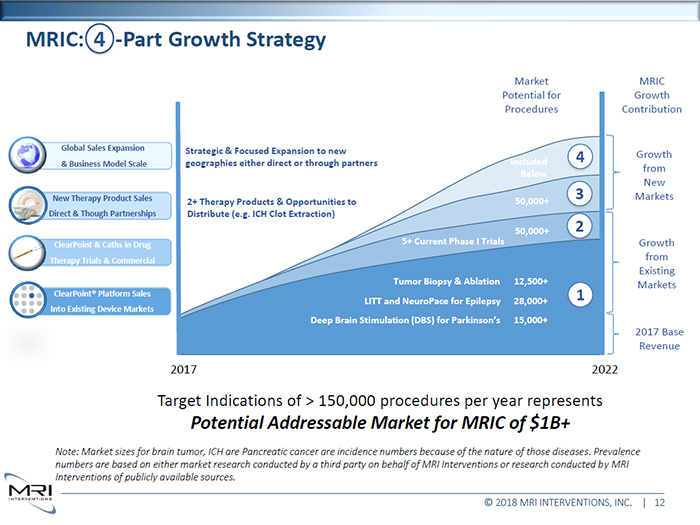
Market Potential for Procedures MRIC Growth Cntribution New Therapy Product Sales Direct & Though Partnerships ClearPoint & Caths in Drug Therapy Trials & Commercial Strategic & Focused Expansion to new geographies either direct or through partners 2+ Therapy Products & Opportunities to Distribute (e.g. ICH Clot Extraction) 5+ Current Phase I Trials Tumor Biopsy & Ablation Included 4 Below 50,000+ 3 50,000+ 2 12,500+ Growth from New Markets Growth from Existing Markets ClearPoint® Platform Sales Into Existing Device Markets LITT and NeuroPace for Epilepsy Deep Brain Stimulation (DBS) for Parkinson’s 28,000+ 1 15,000+ 2017 Base Revenue 2017 2022 Target Indications of > 150,000 procedures per year represents Potential Addressable Market for MRIC of $1B+ Note: Market sizes for brain tumor, ICH are Pancreatic cancer are incidence numbers because of the nature of those diseases. Prevalence numbers are based on either market research conducted by a third party on behalf of MRI Interventions or research conducted by MRI Interventions of publicly available sources.
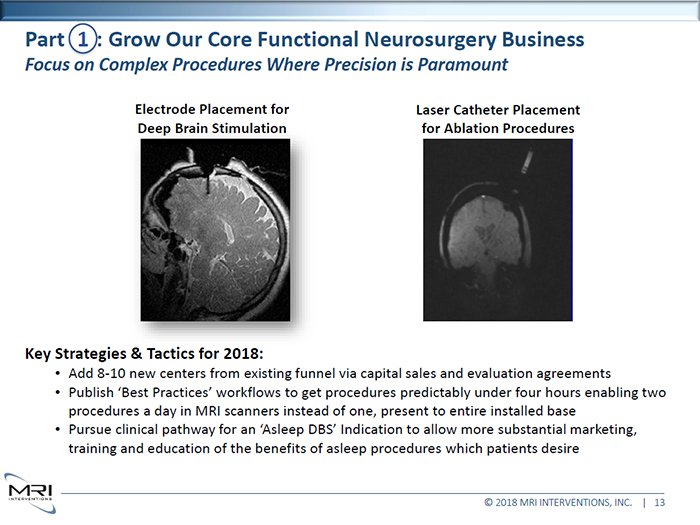
Electrode Placement for Deep Brain Stimulation Laser Catheter Placement for Ablation Procedures Key Strategies & Tactics for 2018: • Add 8-10 new centers from existing funnel via capital sales and evaluation agreements • Publish ‘Best Practices’ workflows to get procedures predictably under four hours enabling two procedures a day in MRI scanners instead of one, present to entire installed base • Pursue clinical pathway for an ‘Asleep DBS’ Indication to allow more substantial marketing, training and education of the benefits of asleep procedures which patients desire
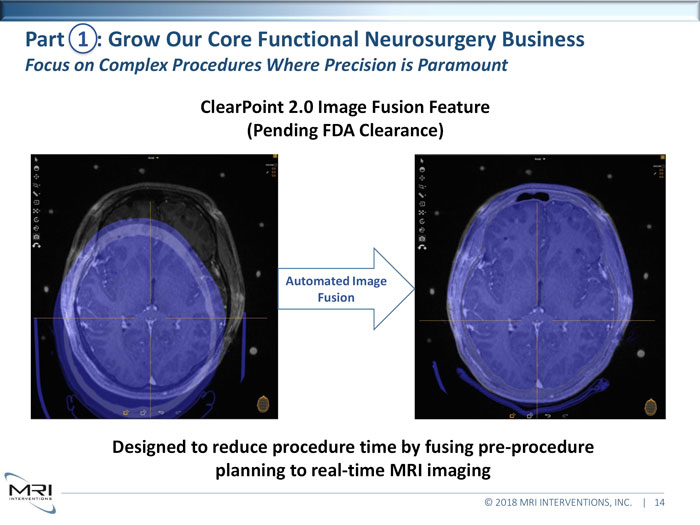
ClearPoint 2.0 Image Fusion Feature (Pending FDA Clearance) Designed to reduce procedure time by fusing pre-procedure planning to real-time MRI imaging
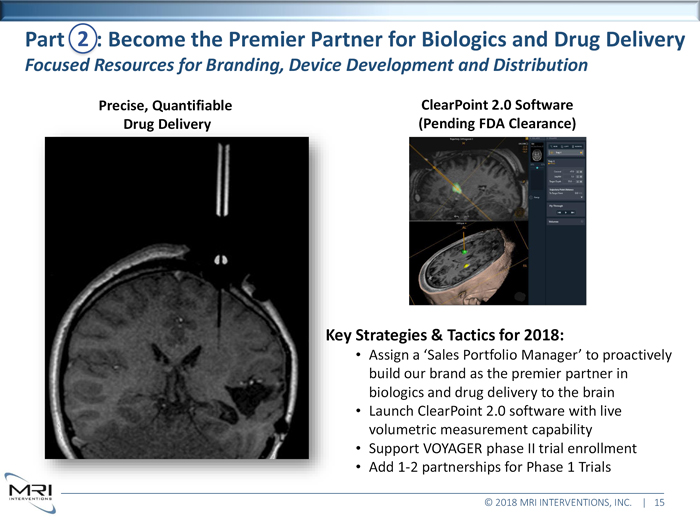
Precise, Quantifiable Drug Delivery ClearPoint 2.0 Software (Pending FDA Clearance)Key Strategies & Tactics for 2018: • Assign a ‘Sales Portfolio Manager’ to proactively build our brand as the premier partner in biologics and drug delivery to the brain • Launch ClearPoint® 2.0 software with live volumetric measurement capability • Begin VOYAGER phase II trial enrollment • Add 1-2 additional partnerships for Phase 1 Trials
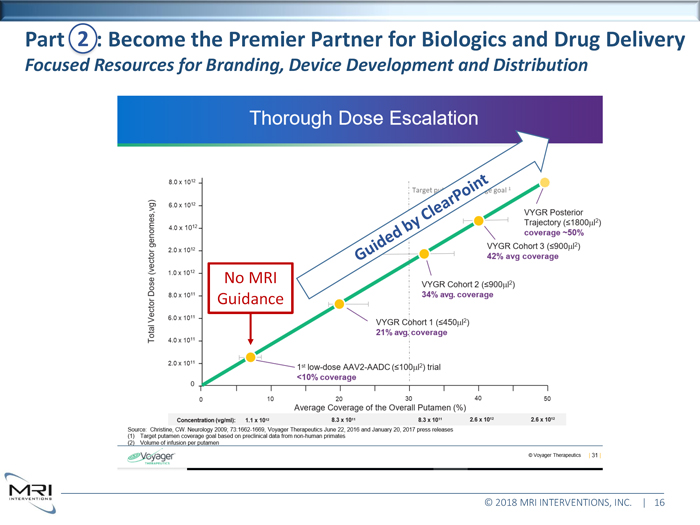
Part 2 Become the Premier Partner for Biologics and Drug Delivery Focused Resources for Branding, Device Development and Distribution No MRI Guidance © 2018 MRI INTERVENTIONS, INC. | 16

Part 2 Become the Premier Partner for Biologics and Drug Delivery Focused Resources for Branding, Device Development and Distribution © 2018 MRI INTERVENTIONS, INC. | 17
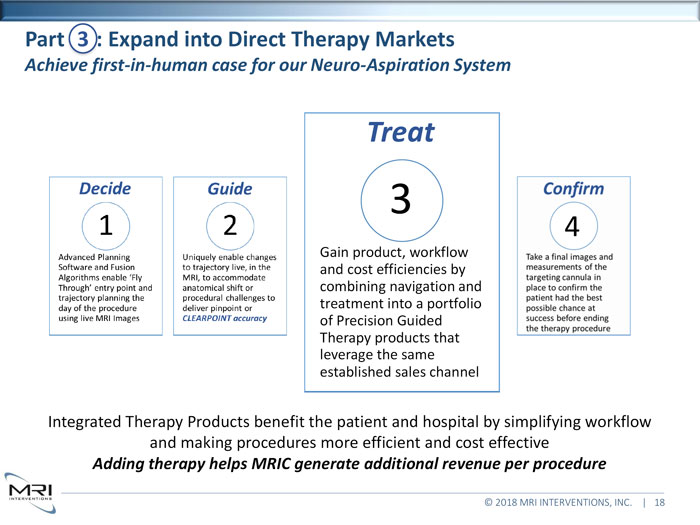
Integrated Therapy Products benefit the patient and hospital by simplifying workflow and making procedures more efficient and cost effective Adding therapy helps MRIC generate additional revenue per procedure

Initial Stroke Product (ClearPoint PURSUIT™) Targets Intracerebral Hemorrhage (ICH) • Collaboration with Mayo Clinic • Only major stroke subtype w/o clearly effective therapy – major unmet medical need • Affects 80,000 to 100,000 people in the US each year Current Approaches for Hemorrhage Removal and Decompression are Inadequate: • Open craniotomy provides visibility but is highly invasive, destroys brain tissue • Minimally invasive approach has very limited ability to quantify volume reduction of the hemorrhage or monitor subsequent bleeds Our Unique ClearPoint PURSUIT™ Approach to ICH • Detailed, continuous, high resolution, 4-D visibility • Minimally invasive approach 4-D Guidance of Neuro Clot Aspiration Key Strategies & Tactics for 2018: • Complete product development for FDA submission in Q3 • Perform first-in-human case by December 2018 • •
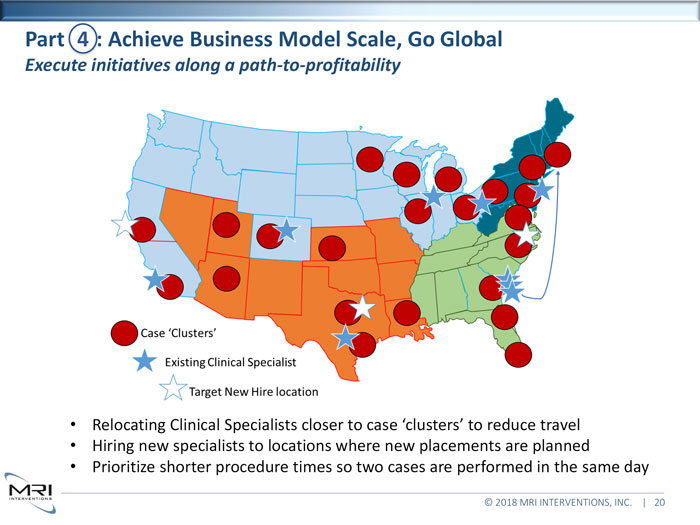
Relocating Clinical Specialists closer to case ‘clusters’ to reduce travel • Hiring new specialists to locations where new placements are planned • Prioritize shorter procedure times so two cases are performed in the same day •
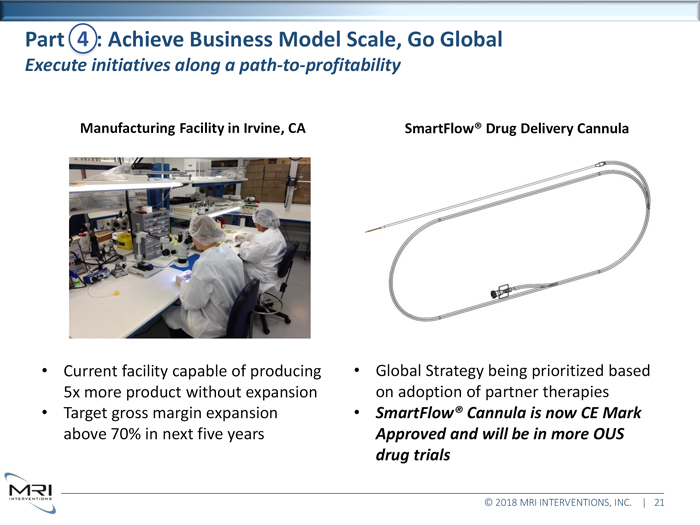
Manufacturing Facility in Irvine, CA • Current facility capable of producing 5x more product without expansion • Target gross margin expansion above 70% in next five years Global Strategy being prioritized based on adoption of partner therapies • SmartFlow® Cannulas is now CE Mark Approved and will be in more OUS drug trials •
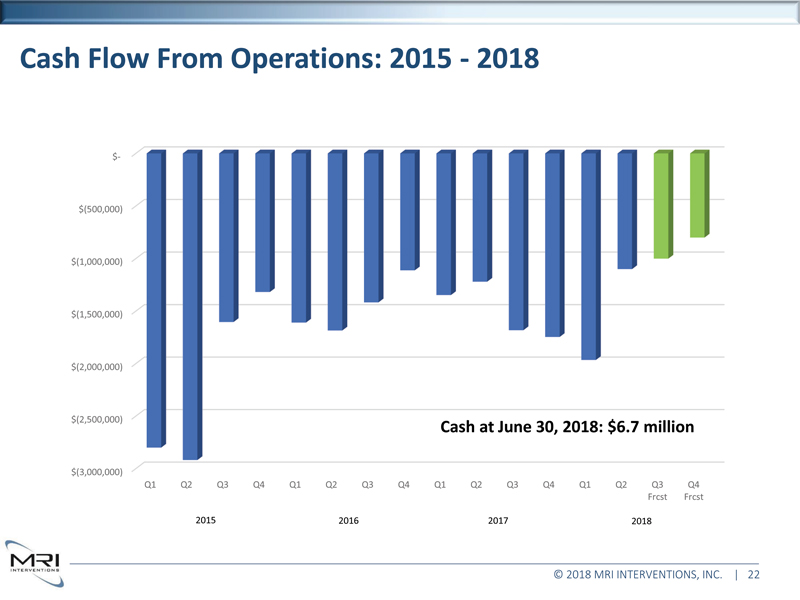
Cash Flow From Operations: 2015 - 2018 $- $(500,000) $(1,000,000) $(1,500,000) $(2,000,000) $(2,500,000) $(3,000,000) Q1 Q2 Q3 Q4 Q1 Q2 Q3 Q4 Q1 Q2 Q3 Q4 Q1 Q2 Frcst Q3 Frcst Q4 Frcst 2015 2016 2017 2018

MRIC: Executive Summary • Unique, Platform technology enabling Precision MRI-Guided Therapies to restore quality of life for some of the most debilitating disorders • Large, Growing installed base in 53 of 250+ leading Neurology centers in U.S. • Procedure volume has grown 43%+ CAGR from 2013-2017 • 80%+ of current revenue from single-use, high-margin disposables • Pipeline of new revenue streams from product improvements, new drug therapy trials, and standalone therapy products • Total potential addressable market > $1B for our products and pipeline • Strong cash position to fund Commercial and R&D initiatives • A passionate team of embedded scientists and specialists